Use the given molar solubilities in pure water to calculate Ksp for each compound. b. Ag2SO3; molar solubility = 1.55⨉10-5 M c. Pd(SCN)2; molar solubility = 2.22⨉10-8 M
Ch.18 - Aqueous Ionic Equilibrium

Chapter 18, Problem 100
The solubility of copper(I) chloride is 3.91 mg per 100.0 mL of solution. Calculate Ksp for CuCl.
 Verified step by step guidance
Verified step by step guidance1
Convert the solubility of copper(I) chloride from mg/100 mL to mol/L by dividing the mass by the molar mass of CuCl and then converting to liters.
Write the dissolution equation for copper(I) chloride: \( \text{CuCl(s)} \rightleftharpoons \text{Cu}^+ (aq) + \text{Cl}^- (aq) \).
Determine the molar concentration of \( \text{Cu}^+ \) and \( \text{Cl}^- \) ions in the solution, which will be equal to the solubility in mol/L since the stoichiometry is 1:1.
Write the expression for the solubility product constant \( K_{sp} \) for CuCl: \( K_{sp} = [\text{Cu}^+][\text{Cl}^-] \).
Substitute the molar concentrations of \( \text{Cu}^+ \) and \( \text{Cl}^- \) into the \( K_{sp} \) expression to calculate the solubility product.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Solubility Product Constant (Ksp)
The solubility product constant (Ksp) is an equilibrium constant that applies to the solubility of sparingly soluble ionic compounds. It is defined as the product of the molar concentrations of the ions, each raised to the power of their coefficients in the balanced equation. For copper(I) chloride (CuCl), Ksp can be calculated from the concentrations of Cu+ and Cl- ions in a saturated solution.
Recommended video:
Guided course

Solubility Product Constant
Dissociation of Ionic Compounds
Ionic compounds dissociate into their constituent ions when dissolved in water. For CuCl, it dissociates into one Cu+ ion and one Cl- ion. Understanding this dissociation is crucial for calculating Ksp, as the concentrations of these ions directly influence the solubility product.
Recommended video:
Guided course

Ionic Compounds Naming
Concentration Calculations
To calculate Ksp, it is essential to convert the solubility from mg per 100.0 mL to molarity (mol/L). This involves determining the molar mass of CuCl and using the solubility value to find the concentration of Cu+ and Cl- ions in the solution. Accurate concentration calculations are fundamental for deriving the Ksp value.
Recommended video:
Guided course
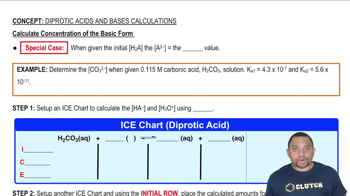
Calculate Concentration of the Basic Form
Related Practice
Textbook Question
Textbook Question
Two compounds with general formulas AX and AX2 have Ksp = 1.5⨉10-5. Which of the two compounds has the higher molar solubility?
Textbook Question
Consider the compounds with the generic formulas listed and their corresponding molar solubilities in pure water. Which compound has the smallest value of Ksp? a. AX; molar solubility = 1.35⨉10-4 M b. AX2; molar solubility = 2.25⨉10-4 M c. A2X; molar solubility = 1.75⨉10-4 M
Textbook Question
Calculate the molar solubility of barium fluoride in each liquid or solution. a. pure water
Textbook Question
Calculate the molar solubility of barium fluoride in each liquid or solution. b. 0.10 M Ba(NO3)2
Textbook Question
Calculate the molar solubility of barium fluoride in each liquid or solution. c. 0.15 M NaF
