Consider the compounds with the generic formulas listed and their corresponding molar solubilities in pure water. Which compound has the smallest value of Ksp? a. AX; molar solubility = 1.35⨉10-4 M b. AX2; molar solubility = 2.25⨉10-4 M c. A2X; molar solubility = 1.75⨉10-4 M
Ch.18 - Aqueous Ionic Equilibrium

Chapter 18, Problem 101b
Calculate the molar solubility of barium fluoride in each liquid or solution. b. 0.10 M Ba(NO3)2
 Verified step by step guidance
Verified step by step guidance1
Write the dissolution equation for barium fluoride (BaF2): BaF2(s) ⇌ Ba2+(aq) + 2F-(aq).
Identify the initial concentrations of the ions in the solution. Since the solution already contains 0.10 M Ba(NO3)2, the initial concentration of Ba2+ is 0.10 M. The initial concentration of F- is 0 M.
Set up the expression for the solubility product constant (Ksp) of BaF2, which is given by Ksp = [Ba2+][F-]2. Let 's' be the molar solubility of BaF2 in the 0.10 M Ba(NO3)2 solution.
Substitute the equilibrium concentrations into the Ksp expression. Since Ba2+ is already present at 0.10 M, the concentration at equilibrium will be 0.10 M + s. The concentration of F- at equilibrium will be 2s.
Solve the equation for 's' to find the molar solubility of BaF2 in the 0.10 M Ba(NO3)2 solution. This involves substituting the expressions for the equilibrium concentrations into the Ksp equation and solving for 's'.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
7mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Molar Solubility
Molar solubility refers to the maximum amount of a solute that can dissolve in a given volume of solvent at a specific temperature, expressed in moles per liter (M). It is a crucial concept in understanding how ionic compounds dissociate in solution and is often determined through equilibrium expressions.
Recommended video:
Guided course
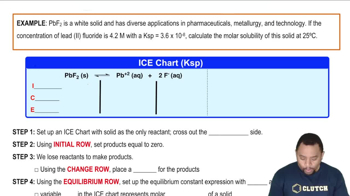
Molar Solubility Example
Common Ion Effect
The common ion effect describes the decrease in solubility of an ionic compound when a common ion is added to the solution. In this case, the presence of Ba<sup>2+</sup> ions from Ba(NO<sub>3</sub>)<sub>2</sub> will shift the equilibrium of barium fluoride dissolution, reducing its molar solubility due to Le Chatelier's principle.
Recommended video:
Guided course

Common Ion Effect
Equilibrium Constant (Ksp)
The solubility product constant (Ksp) is an equilibrium constant that applies to the dissolution of sparingly soluble ionic compounds. It quantifies the product of the molar concentrations of the ions in a saturated solution, raised to the power of their coefficients in the balanced dissolution equation, and is essential for calculating molar solubility in different solutions.
Recommended video:
Guided course

Equilibrium Constant K
Related Practice
Textbook Question
Textbook Question
The solubility of copper(I) chloride is 3.91 mg per 100.0 mL of solution. Calculate Ksp for CuCl.
Textbook Question
Calculate the molar solubility of barium fluoride in each liquid or solution. a. pure water
Textbook Question
Calculate the molar solubility of barium fluoride in each liquid or solution. c. 0.15 M NaF
Textbook Question
Calculate the molar solubility of MX (Ksp = 1.27⨉10-36) in each liquid or solution. b. 0.25 M MCl2
Textbook Question
Calculate the molar solubility of MX (Ksp = 1.27⨉10-36) in each liquid or solution. c. 0.20 M Na2X
