The activation barrier for the hydrolysis of sucrose into glucose and fructose is 108 kJ/mol. If an enzyme increases the rate of the hydrolysis reaction by a factor of 1 million, how much lower must the activation barrier be when sucrose is in the active site of the enzyme? (Assume that the frequency factors for the catalyzed and uncatalyzed reactions are identical and a temperature of 25 °C.)
Ch.15 - Chemical Kinetics

Chapter 15, Problem 89a
The tabulated data were collected for this reaction at 500 °C: CH3CN(g) → CH3NC( g) a. Determine the order of the reaction and the value of the rate constant at this temperature.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the problem. We need to determine the order of the reaction and the rate constant for the given reaction at 500 °C.
Step 2: Analyze the data. Look at the concentration of CH3CN over time to see how it changes. This will help us determine the order of the reaction.
Step 3: Determine the reaction order. Use the method of initial rates or plot concentration vs. time data to find if the reaction is zero, first, or second order.
Step 4: Calculate the rate constant. Once the order is known, use the appropriate rate law equation to calculate the rate constant, k.
Step 5: Verify your results. Check if the calculated rate constant and reaction order are consistent with the data provided.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
5mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Reaction Order
The order of a reaction refers to the power to which the concentration of a reactant is raised in the rate law. It indicates how the rate of reaction depends on the concentration of reactants. For example, a first-order reaction has a rate that is directly proportional to the concentration of one reactant, while a second-order reaction depends on the square of the concentration of one reactant or the product of the concentrations of two reactants.
Recommended video:
Guided course

Average Bond Order
Rate Constant (k)
The rate constant, denoted as 'k', is a proportionality factor in the rate law that relates the rate of a reaction to the concentrations of the reactants. It is specific to a given reaction at a particular temperature and provides insight into the speed of the reaction. The value of 'k' can be determined experimentally and varies with temperature, reflecting the energy barrier that must be overcome for the reaction to proceed.
Recommended video:
Guided course

Equilibrium Constant K
Arrhenius Equation
The Arrhenius equation describes how the rate constant 'k' changes with temperature. It is expressed as k = A * e^(-Ea/RT), where 'A' is the pre-exponential factor, 'Ea' is the activation energy, 'R' is the universal gas constant, and 'T' is the temperature in Kelvin. This equation highlights the exponential relationship between temperature and reaction rate, indicating that higher temperatures generally lead to increased reaction rates due to more molecules having sufficient energy to overcome the activation energy barrier.
Recommended video:
Guided course
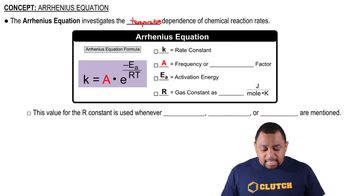
Arrhenius Equation
Related Practice
Textbook Question
Textbook Question
The tabulated data were collected for this reaction at 500 °C: CH3CN(g) → CH3NC( g) b. What is the half-life for this reaction (at the initial concentration)?
Textbook Question
The tabulated data were collected for this reaction at a certain temperature: X2Y → 2 X + Y a. Determine the order of the reaction and the value of the rate constant at this temperature.
Textbook Question
The tabulated data were collected for this reaction at a certain temperature: X2Y → 2 X + Y c. What is the concentration of X after 10.0 hours?
