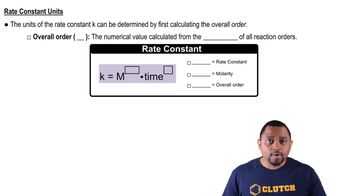
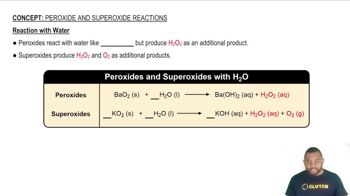
Gas Collection Over Water
When collecting gas over water, the total pressure of the gas collected is the sum of the partial pressure of the gas and the vapor pressure of water. In this scenario, the barometric pressure is 742.5 mmHg, and the vapor pressure of water at 20.0 °C is 17.5 mmHg. Understanding this concept is vital for accurately calculating the volume of O2 produced, as it allows for the adjustment of the measured pressure to account for the water vapor.

 Verified step by step guidance
Verified step by step guidance