Iodine atoms combine to form I2 in liquid hexane solvent with a rate constant of 1.5⨉1010 L/mols. The reaction is second order in I. Since the reaction occurs so quickly, the only way to study the reaction is to create iodine atoms almost instantaneously, usually by photochemical decomposition of I2. Suppose a flash of light creates an initial [I] concentration of 0.0100 M. How long will it take for 95% of the newly created iodine atoms to recombine to form I2?
Ch.15 - Chemical Kinetics

Chapter 15, Problem 101a
Consider this energy diagram:
a. How many elementary steps are involved in this reaction?
 Verified step by step guidance
Verified step by step guidance1
Identify the number of peaks in the energy diagram. Each peak represents a transition state, which corresponds to an elementary step.
Count the number of valleys or intermediates between the peaks. These valleys represent the intermediates formed during the reaction.
The number of elementary steps is equal to the number of peaks in the energy diagram.
Verify that each peak corresponds to a distinct transition state, indicating a separate elementary step.
Summarize the total number of elementary steps based on the number of peaks identified in the energy diagram.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Elementary Steps
Elementary steps are the individual reactions that occur in a multi-step chemical reaction. Each step represents a single molecular event, such as the collision of reactants leading to products. Understanding the number of elementary steps is crucial for analyzing reaction mechanisms and determining the overall rate of the reaction.
Recommended video:
Guided course

Reaction Mechanism Overview
Energy Diagram
An energy diagram visually represents the energy changes that occur during a chemical reaction. It typically shows the energy of reactants, products, and the transition states, indicating the activation energy required for the reaction to proceed. By examining the diagram, one can identify the number of steps and the energy barriers involved in the reaction.
Recommended video:
Guided course
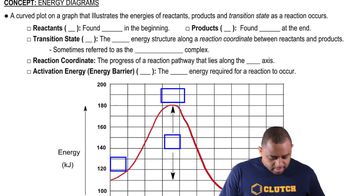
Energy Diagrams
Reaction Mechanism
A reaction mechanism is a detailed description of the step-by-step process by which reactants are converted into products. It includes the sequence of elementary steps, intermediates formed, and the transition states. Understanding the mechanism is essential for predicting reaction rates and the influence of various factors on the reaction.
Recommended video:
Guided course

Reaction Mechanism Overview
Related Practice
Textbook Question
Textbook Question
Consider this energy diagram:
d. Is the overall reaction endothermic or exothermic?
Textbook Question
Consider the reaction in which HCl adds across the double bond of ethene: HCl + H2C=CH2 → H3C-CH2Cl The following mechanism, with the accompanying energy diagram, has been suggested for this reaction:
Step 1 HCl + H2C=CH2 → H3C=CH2+ + Cl-
Step 2 H3C=CH2+ + Cl- → H3C-CH2Cl
a. Based on the energy diagram, determine which step is rate limiting.
