What mass of salt (NaCl) should you add to 1.00 L of water in an ice cream maker to make a solution that freezes at -10.0 °C? Assume complete dissociation of the NaCl and density of 1.00 g/mL for water.
Ch.14 - Solutions

Chapter 14, Problem 101
A 0.100 M ionic solution has an osmotic pressure of 8.3 atm at 25 °C. Calculate the van't Hoff factor (i) for this solution.
 Verified step by step guidance
Verified step by step guidance1
Identify the formula for osmotic pressure, which is given by \(\Pi = iMRT\), where \(\Pi\) is the osmotic pressure, \(i\) is the van't Hoff factor, \(M\) is the molarity of the solution, \(R\) is the ideal gas constant, and \(T\) is the temperature in Kelvin.
Convert the temperature from Celsius to Kelvin by adding 273.15 to the Celsius temperature. For this problem, convert 25 °C to Kelvin.
Use the value of the ideal gas constant \(R\) that is appropriate for the units of pressure in atmospheres, which is approximately 0.0821 L·atm/mol·K.
Rearrange the osmotic pressure formula to solve for the van't Hoff factor \(i\). The rearranged formula is \(i = \frac{\Pi}{MRT}\).
Substitute the values of \(\Pi\), \(M\), \(R\), and \(T\) into the rearranged formula to calculate the van't Hoff factor \(i\).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Osmotic Pressure
Osmotic pressure is the pressure required to prevent the flow of solvent into a solution through a semipermeable membrane. It is directly proportional to the concentration of solute particles in the solution and can be calculated using the formula π = iCRT, where π is the osmotic pressure, i is the van't Hoff factor, C is the molarity of the solution, and R is the ideal gas constant.
Recommended video:
Guided course
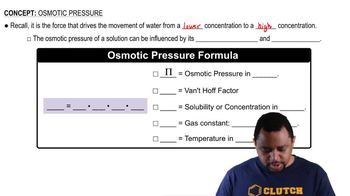
Osmotic Pressure Formula
van't Hoff Factor (i)
The van't Hoff factor (i) represents the number of particles into which a solute dissociates in solution. For ionic compounds, this factor can be greater than one, as they separate into multiple ions. For example, NaCl dissociates into Na⁺ and Cl⁻, giving it a van't Hoff factor of 2. Understanding this concept is crucial for calculating colligative properties like osmotic pressure.
Recommended video:
Guided course
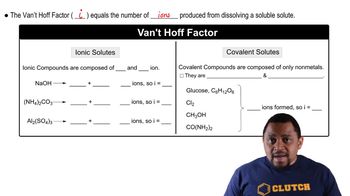
Van't Hoff Factor
Colligative Properties
Colligative properties are properties of solutions that depend on the number of solute particles rather than their identity. These properties include boiling point elevation, freezing point depression, vapor pressure lowering, and osmotic pressure. The van't Hoff factor plays a significant role in these properties, as it affects the effective concentration of solute particles in the solution.
Recommended video:
Guided course

Colligative Properties
Related Practice
Textbook Question
1
views
Textbook Question
Use the van't Hoff factors in Table 13.9 to calculate each colligative property: a. the melting point of a 0.100 m iron(III) chloride solution
Textbook Question
A 1.2 m aqueous solution of an ionic compound with the formula MX2 has a boiling point of 101.4 °C. Calculate the van't Hoff factor (i) for MX2 at this concentration.
Textbook Question
Calculate the vapor pressure at 25 °C of an aqueous solution that is 5.50% NaCl by mass. (Assume complete dissociation of the solute.)
Textbook Question
An aqueous CaCl2 solution has a vapor pressure of 81.6 mmHg at 50 °C. The vapor pressure of pure water at this temperature is 92.6 mmHg. What is the concentration of CaCl2 in mass percent? (Assume complete dissociation of the solute.)
