Textbook Question
Explain the observed trend in the melting points of the hydrogen halides.
HI -50.8 °C
HBr -88.5 °C
HCl -114.8 °C
HF -83.1 °C
1
views

 Verified step by step guidance
Verified step by step guidance
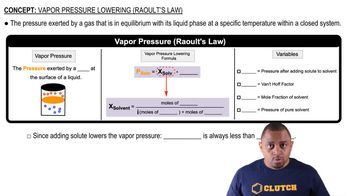

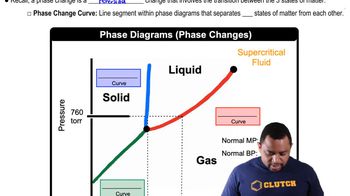
Explain the observed trend in the melting points of the hydrogen halides.
HI -50.8 °C
HBr -88.5 °C
HCl -114.8 °C
HF -83.1 °C
Explain the observed trend in the boiling points of these compounds.
H2Te -2 °C
H2Se -41.5 °C
H2S -60.7 °C
H2O 100 °C
The vapor pressure of CCl3F at 300 K is 856 torr. If 3.5 g of CCl3F is enclosed in a 1.0-L container, will any liquid be present? If so, what mass of liquid?
A sample of steam with a mass of 0.552 g and at a temperature of 100 °C condenses into an insulated container holding 4.25 g of water at 5.0 °C. Assuming that no heat is lost to the surroundings, what is the final temperature of the mixture?