A cylinder with a moveable piston contains 0.553 mol of gas and has a volume of 253 mL. What is its volume if an additional 0.365 mol of gas is added to the cylinder? (Assume constant temperature and pressure.)
Ch.6 - Gases

Chapter 6, Problem 34
A syringe containing 1.55 mL of oxygen gas is cooled from 95.3 °C to 0.0 °C. What is the final volume of oxygen gas?
 Verified step by step guidance
Verified step by step guidance1
Identify the initial and final temperatures: \( T_1 = 95.3 \, ^\circ\text{C} \) and \( T_2 = 0.0 \, ^\circ\text{C} \). Convert these temperatures to Kelvin by adding 273.15 to each.
Use Charles's Law, which states that \( \frac{V_1}{T_1} = \frac{V_2}{T_2} \), where \( V_1 \) and \( V_2 \) are the initial and final volumes, and \( T_1 \) and \( T_2 \) are the initial and final temperatures in Kelvin.
Substitute the known values into Charles's Law: \( V_1 = 1.55 \, \text{mL} \), \( T_1 = 95.3 + 273.15 \, \text{K} \), and \( T_2 = 0.0 + 273.15 \, \text{K} \).
Rearrange the equation to solve for \( V_2 \): \( V_2 = V_1 \times \frac{T_2}{T_1} \).
Calculate \( V_2 \) using the rearranged equation to find the final volume of the oxygen gas.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Was this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Ideal Gas Law
The Ideal Gas Law relates the pressure, volume, temperature, and number of moles of a gas through the equation PV = nRT. This law assumes that gases behave ideally, meaning they occupy no volume and have no intermolecular forces. Understanding this relationship is crucial for solving problems involving gas behavior under changing conditions.
Recommended video:
Guided course

Ideal Gas Law Formula
Charles's Law
Charles's Law states that the volume of a gas is directly proportional to its temperature (in Kelvin) when pressure is held constant. This means that as the temperature of a gas decreases, its volume also decreases. This principle is essential for calculating the final volume of gas when the temperature changes, as in the given question.
Recommended video:
Guided course
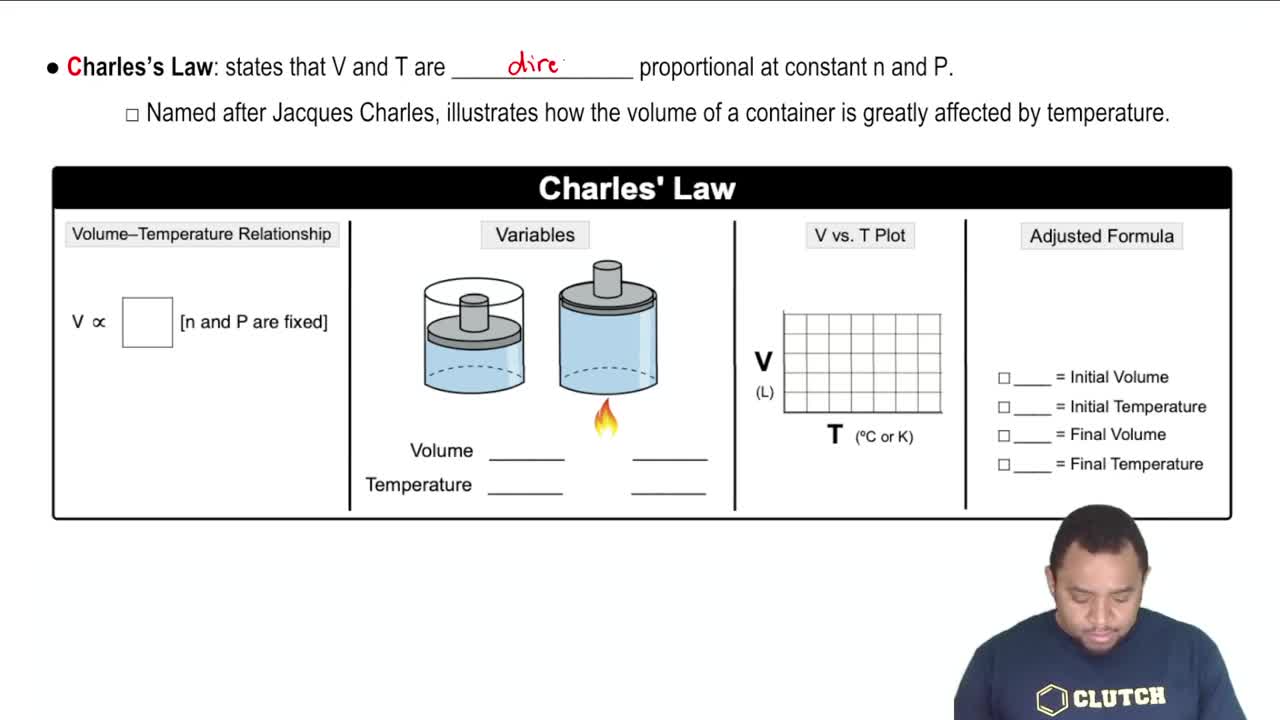
Charles's Law
Temperature Conversion
In gas law calculations, temperatures must be expressed in Kelvin rather than Celsius. The conversion from Celsius to Kelvin is done by adding 273.15 to the Celsius temperature. This conversion is necessary to ensure accurate calculations when applying gas laws, as they require absolute temperature values.
Recommended video:
Guided course
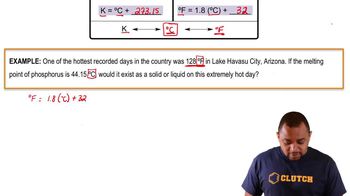
Temperature Conversion Example
Related Practice
Textbook Question
Textbook Question
A sample of gas has an initial volume of 5.6 L at a pressure of 735 mmHg. If the volume of the gas is increased to 9.4 L, what is its pressure?
1
views
Textbook Question
A sample of gas has an initial volume of 13.9 L at a pressure of 1.22 atm. If the sample is compressed to a volume of 10.3 L, what is its pressure?
1
views
Textbook Question
A 48.3-mL sample of gas in a cylinder is warmed from 22 °C to 87 °C. What is its volume at the final temperature?
1
views
Textbook Question
A balloon contains 0.158 mol of gas and has a volume of 2.46 L. If an additional 0.113 mol of gas is added to the balloon (at the same temperature and pressure), what is its final volume?
2
views
Textbook Question
What volume is occupied by 0.118 mol of helium gas at a pressure of 0.97 atm and a temperature of 305 K?
