A cylinder with a moveable piston contains 0.553 mol of gas and has a volume of 253 mL. What is its volume if an additional 0.365 mol of gas is added to the cylinder? (Assume constant temperature and pressure.)
Ch.6 - Gases

Chapter 6, Problem 33
A 48.3-mL sample of gas in a cylinder is warmed from 22 °C to 87 °C. What is its volume at the final temperature?
 Verified step by step guidance
Verified step by step guidance1
Identify the initial and final temperatures and convert them from Celsius to Kelvin. Use the formula: \( T(K) = T(°C) + 273.15 \).
Apply Charles's Law, which states that \( \frac{V_1}{T_1} = \frac{V_2}{T_2} \), where \( V_1 \) and \( T_1 \) are the initial volume and temperature, and \( V_2 \) and \( T_2 \) are the final volume and temperature.
Substitute the known values into the equation: \( V_1 = 48.3 \text{ mL} \), \( T_1 = 22 + 273.15 \text{ K} \), and \( T_2 = 87 + 273.15 \text{ K} \).
Rearrange the equation to solve for \( V_2 \): \( V_2 = V_1 \times \frac{T_2}{T_1} \).
Calculate \( V_2 \) using the rearranged equation to find the final volume of the gas at 87 °C.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Was this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Charles's Law
Charles's Law states that the volume of a gas is directly proportional to its temperature (in Kelvin) when pressure is held constant. This relationship can be expressed mathematically as V1/T1 = V2/T2, where V is volume and T is temperature. Understanding this law is essential for solving problems involving changes in temperature and volume of gases.
Recommended video:
Guided course
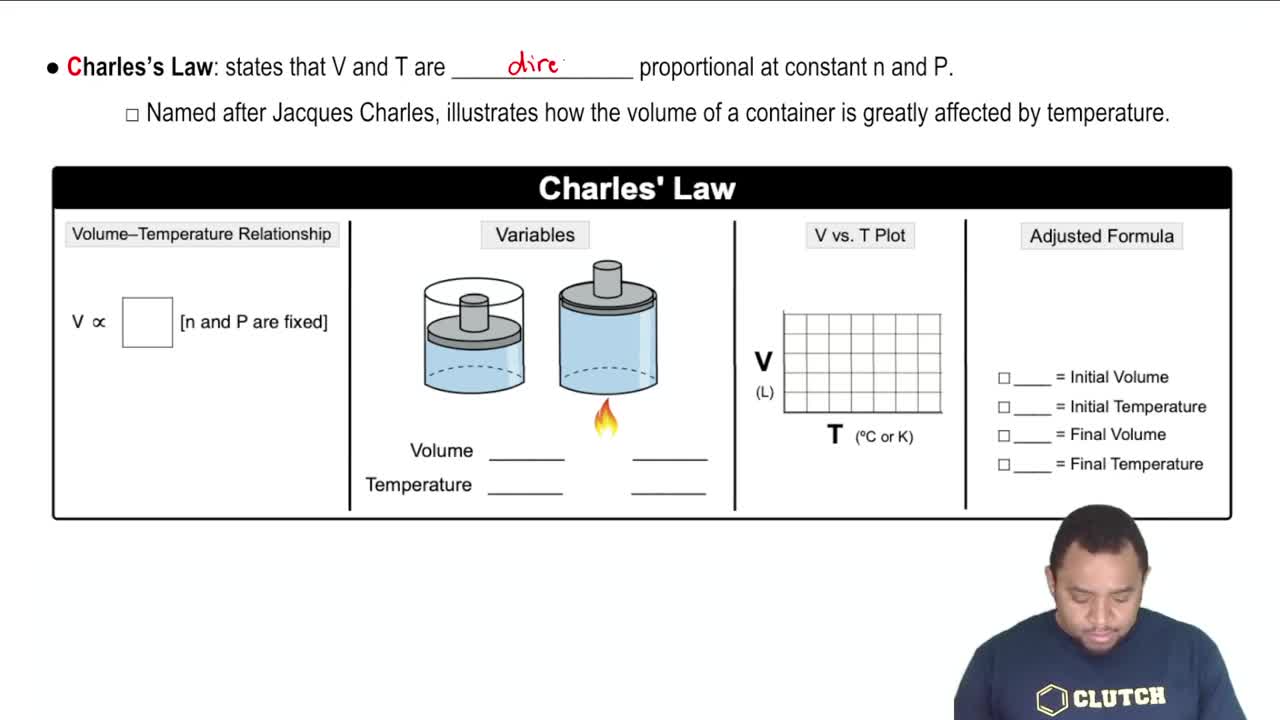
Charles's Law
Temperature Conversion
In gas law calculations, temperatures must be expressed in Kelvin rather than Celsius. The conversion from Celsius to Kelvin is done by adding 273.15 to the Celsius temperature. This step is crucial because gas laws are based on absolute temperature, which affects the behavior of gases.
Recommended video:
Guided course
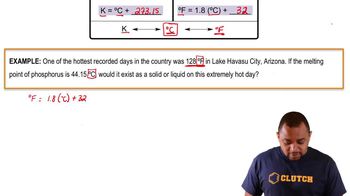
Temperature Conversion Example
Ideal Gas Behavior
The ideal gas law describes the behavior of an ideal gas, which follows the relationships defined by pressure, volume, temperature, and the number of moles of gas. While real gases may deviate from ideal behavior under certain conditions, understanding this concept helps in applying gas laws effectively and predicting how gases will respond to changes in temperature and volume.
Recommended video:
Guided course

Ideal Gas Law Formula
Related Practice
Textbook Question
Textbook Question
Given a barometric pressure of 751.5 mmHg, calculate the pressure of each gas sample as indicated by the manometer.
(b)
Textbook Question
A sample of gas has an initial volume of 5.6 L at a pressure of 735 mmHg. If the volume of the gas is increased to 9.4 L, what is its pressure?
1
views
Textbook Question
A sample of gas has an initial volume of 13.9 L at a pressure of 1.22 atm. If the sample is compressed to a volume of 10.3 L, what is its pressure?
1
views
Textbook Question
A syringe containing 1.55 mL of oxygen gas is cooled from 95.3 °C to 0.0 °C. What is the final volume of oxygen gas?
2
views
Textbook Question
A balloon contains 0.158 mol of gas and has a volume of 2.46 L. If an additional 0.113 mol of gas is added to the balloon (at the same temperature and pressure), what is its final volume?
2
views
