Textbook Question
Determine whether or not each element is a main-group element. a. tellurium b. potassium c. vanadium d. manganese
1
views

 Verified step by step guidance
Verified step by step guidance

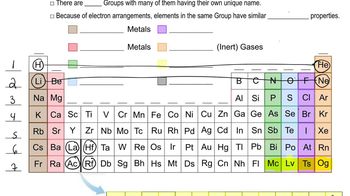
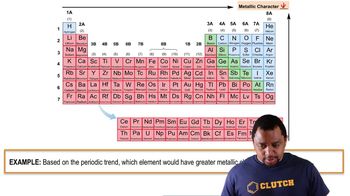
Determine whether or not each element is a main-group element. a. tellurium b. potassium c. vanadium d. manganese
Determine whether or not each element is a transition element. a. Cr b. Br c. Mo d. Cs
Classify each element as an alkali metal, alkaline earth metal, halogen, or noble gas. a. F b. Sr c. K d. Ne e. At
The atomic mass of fluorine is 18.998 amu, and its mass spectrum shows a large peak at this mass. The atomic mass of chlorine is 35.45 amu, yet the mass spectrum of chlorine does not show a peak at this mass. Explain the difference.