Textbook Question
Fill in the blanks to complete the table.
Symbol Ion Formed Number of Electrons in Ion Number of Protons in Ion
Cl ______ ______ 17
Te ______ 54 ______
Br Br– ______ ______
______ Sr2+ ______ 38
1
views

 Verified step by step guidance
Verified step by step guidance
Fill in the blanks to complete the table.
Symbol Ion Formed Number of Electrons in Ion Number of Protons in Ion
Cl ______ ______ 17
Te ______ 54 ______
Br Br– ______ ______
______ Sr2+ ______ 38
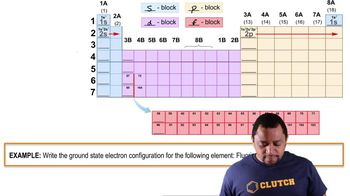
Write the symbol for each element and classify it as a metal, nonmetal, or metalloid. a. gold b. fluorine c. sodium d. tin e. argon
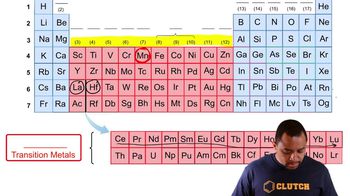
Determine whether or not each element is a main-group element. a. tellurium b. potassium c. vanadium d. manganese
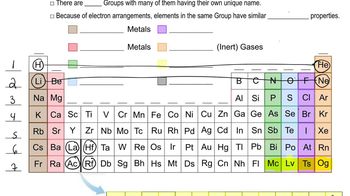
Classify each element as an alkali metal, alkaline earth metal, halogen, or noble gas. a. F b. Sr c. K d. Ne e. At
Which pair of elements do you expect to be most similar? Why? a. nitrogen and oxygen b. titanium and gallium c. lithium and sodium d. germanium and arsenic e. argon and bromine