A battery relies on the oxidation of magnesium and the reduction of Cu2+. The initial concentrations of Mg2+ and Cu2+ are 1.0 × 10–4 M and 1.5 M, respectively, in 1.0-liter half-cells. b. What is the voltage of the battery after delivering 5.0 A for 8.0 h?

A rechargeable battery is constructed based on a concentration cell constructed of two Ag/Ag+ half-cells. The volume of each half-cell is 2.0 L, and the concentrations of Ag+ in the half-cells are 1.25 M and 1.0 × 10–3 M. b. What mass of silver is plated onto the cathode by running at 3.5 A for 5.5 h?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Electrochemical Cells

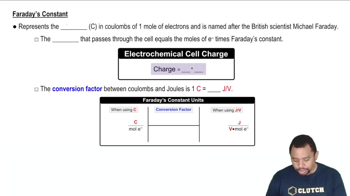
Faraday's Law of Electrolysis
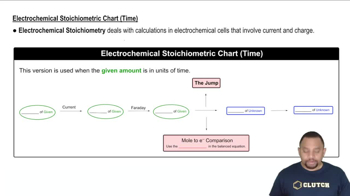
Current and Time Relationship
A battery relies on the oxidation of magnesium and the reduction of Cu2+. The initial concentrations of Mg2+ and Cu2+ are 1.0 × 10–4 M and 1.5 M, respectively, in 1.0-liter half-cells. c. How long can the battery deliver 5.0 A before going dead?
A rechargeable battery is constructed based on a concentration cell constructed of two Ag/Ag+ half-cells. The volume of each half-cell is 2.0 L, and the concentrations of Ag+ in the half-cells are 1.25 M and 1.0×10–3 M. a. How long can this battery deliver 2.5 Aof current before it goes dead?
A rechargeable battery is constructed based on a concentration cell constructed of two Ag/Ag+ half-cells. The volume of each half-cell is 2.0 L, and the concentrations of Ag+ in the half-cells are 1.25 M and 1.0 × 10–3 M. c. Upon recharging, how long would it take to redissolve 1.00 × 102 g of silver at a charging current of 10.0 amps?
