A 5.55-g sample of a weak acid with Ka = 1.3⨉10-4 was combined with 5.00 mL of 6.00 M NaOH, and the resulting solution was diluted to 750.0 mL. The measured pH of the solution was 4.25. What is the molar mass of the weak acid?
Ch.18 - Aqueous Ionic Equilibrium

Chapter 18, Problem 125
If a hard water solution is saturated with calcium carbonate, what volume of the solution has to evaporate to deposit 1.00 × 10^2 mg of CaCO3, given that one of the main components of hard water is CaCO3, and when hard water evaporates, some of the CaCO3 is left behind as a white mineral deposit?
 Verified step by step guidance
Verified step by step guidance1
Identify the molar mass of calcium carbonate (CaCO_3) by adding the atomic masses of calcium (Ca), carbon (C), and oxygen (O).
Convert the mass of CaCO_3 (1.00 \times 10^2 \text{ mg}) to grams by dividing by 1000.
Calculate the number of moles of CaCO_3 using the formula: \text{moles} = \frac{\text{mass in grams}}{\text{molar mass}}.
Determine the concentration of CaCO_3 in the saturated solution, which is typically given or can be found in a reference table.
Use the concentration to find the volume of solution needed to contain the calculated moles of CaCO_3, using the formula: \text{volume} = \frac{\text{moles}}{\text{concentration}}.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Saturation and Solubility
Saturation refers to the point at which a solution can no longer dissolve additional solute at a given temperature and pressure. The solubility of a substance, like calcium carbonate (CaCO3), indicates how much of it can dissolve in a specific volume of solvent. Understanding these concepts is crucial for determining how much of the solution must evaporate to reach a point where CaCO3 precipitates out.
Recommended video:
Guided course
Solubility Rules
Evaporation and Concentration
Evaporation is the process by which liquid turns into vapor, leading to a decrease in the volume of the solution. As water evaporates, the concentration of dissolved substances, such as CaCO3, increases. This concept is essential for calculating how much of the solution must evaporate to achieve the desired concentration that allows for the deposition of a specific mass of CaCO3.
Recommended video:
Guided course
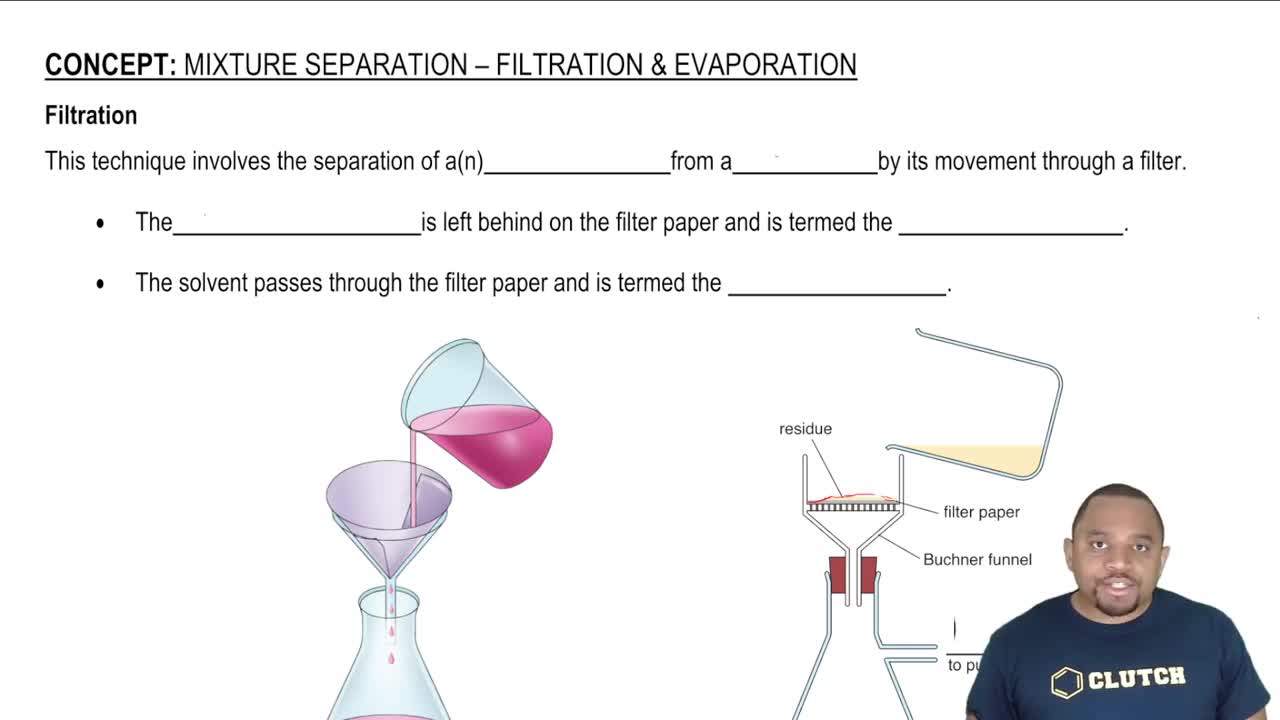
Filtration and Evaporation
Mass-Volume Relationships
Mass-volume relationships involve understanding how the mass of a solute relates to the volume of the solution in which it is dissolved. In this context, knowing the mass of CaCO3 to be deposited allows for the calculation of the volume of solution that must evaporate, based on the concentration of CaCO3 in the saturated solution. This relationship is fundamental for solving the problem presented.
Recommended video:
Guided course

Relationship of Volume and Moles Example
Related Practice
Textbook Question
Textbook Question
Pseudogout, a condition with symptoms similar to those of gout (see Problem 126), is caused by the formation of calcium diphosphate (Ca2P2O7) crystals within tendons, cartilage, and ligaments. Calcium diphosphate will precipitate out of blood plasma when diphosphate levels become abnormally high. If the calcium concentration in blood plasma is 9.2 mg/dL, and Ksp for calcium diphosphate is 8.64⨉10-13, what minimum concentration of diphosphate results in precipitation?
Textbook Question
Calculate the solubility of silver chloride in a solution that is 0.100 M in NH3.
