Textbook Question
Calculate the percent ionization of a formic acid solution having the given concentration. d. 0.0500 M

 Verified step by step guidance
Verified step by step guidance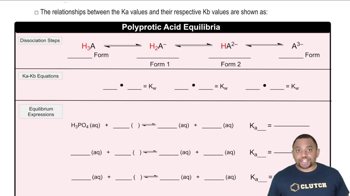

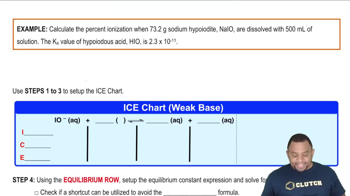
Calculate the percent ionization of a formic acid solution having the given concentration. d. 0.0500 M
A 0.148 M solution of a monoprotic acid has a percent ionization of 1.55%. Determine the acid ionization constant (Ka) for the acid.
Find the pH of each mixture of acids. a. 0.115 M in HBr and 0.125 M in HCHO2