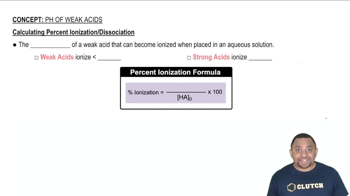
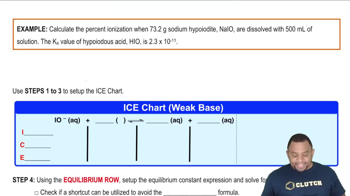
Weak Acid Ionization
Weak acids do not completely dissociate in solution, which means only a fraction of the acid molecules release protons (H+). The extent of this ionization is quantified by the acid dissociation constant (Ka), which indicates the strength of the acid. A lower Ka value corresponds to a weaker acid and less ionization, while a higher Ka indicates a stronger acid with greater ionization.

 Verified step by step guidance
Verified step by step guidance