Textbook Question
The AIDS drug zalcitabine (also known as ddC) is a weak base with a pKb of 9.8. What percentage of the base is protonated in an aqueous zalcitabine solution containing 565 mg/L?

 Verified step by step guidance
Verified step by step guidance
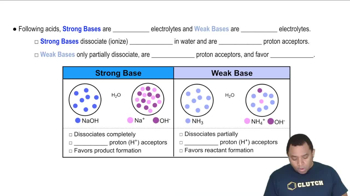

The AIDS drug zalcitabine (also known as ddC) is a weak base with a pKb of 9.8. What percentage of the base is protonated in an aqueous zalcitabine solution containing 565 mg/L?
Determine the pH of each two-component solution. d. 0.088 M HClO4 and 0.022 M KOH
Write net ionic equations for the reactions that take place when aqueous solutions of the following substances are mixed: a. sodium cyanide and nitric acid b. ammonium chloride and sodium hydroxide c. sodium cyanide and ammonium bromide d. potassium hydrogen sulfate and lithium acetate e. sodium hypochlorite and ammonia