Textbook Question
Determine whether each cation is acidic or pH-neutral. For those cations that are acidic, write an equation that shows how the cation acts as an acid. d. CH2NH3+

 Verified step by step guidance
Verified step by step guidance
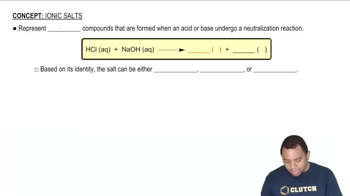
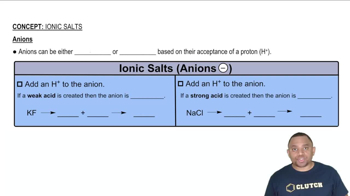

Determine whether each cation is acidic or pH-neutral. For those cations that are acidic, write an equation that shows how the cation acts as an acid. d. CH2NH3+
Determine whether each cation is acidic or pH-neutral. For each cation that is acidic, write an equation that shows how the cation acts as an acid. d. Li+
Determine if each salt will form a solution that is acidic, basic, or pH-neutral. a. Al(NO3)3
Arrange the solutions in order of increasing acidity. NaCl, NH4Cl, NaHCO3, NH4ClO2, NaOH