A reaction in which A, B, and C react to form products is zero order in A, one-half order in B, and second order in C. a. Write a rate law for the reaction.

The tabulated data were collected for this reaction: 2 NO2(g) + F2(g) → 2 NO2F(g). Write an expression for the reaction rate law and calculate the value of the rate constant, k. What is the overall order of the reaction?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Rate Law
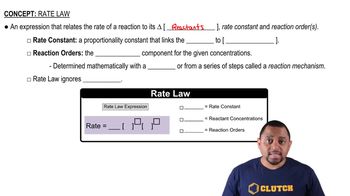
Rate Constant (k)

Overall Order of Reaction

A reaction in which A, B, and C react to form products is zero order in A, one-half order in B, and second order in C. c. By what factor does the reaction rate change if [A] is doubled (and the other reactant concentrations are held constant)? d. By what factor does the reaction rate change if [B] is doubled? e. By what factor does the reaction rate change if [C] is doubled? f. By what factor does the reaction rate change if [C] is doubled (and the other reactant concentrations are held constant)?
Consider the tabulated data showing the initial rate of a reaction (A → products) at several different concentrations of A. What is the order of the reaction? Write a rate law for the reaction including the value of the rate constant, k.
The tabulated data were collected for this reaction: CH3Cl(g) + 3 Cl2(g) → CCl4( g) + 3 HCl(g)
Write an expression for the reaction rate law and calculate the value of the rate constant, k. What is the overall order of the reaction?
Indicate the order of reaction consistent with each observation.
a. The half-life of the reaction gets shorter as the initial concentration is increased.
b. A plot of the natural log of the concentration of the reactant versus time yields a straight line.
Indicate the order of reaction consistent with each observation c. The half-life of the reaction gets longer as the initial concentration is increased.
