Textbook Question
Calculate the freezing point and boiling point of each aqueous solution, assuming complete dissociation of the solute. a. 0.100 m K2S b. 21.5 g of CuCl2 in 4.50⨉102 g water

 Verified step by step guidance
Verified step by step guidance
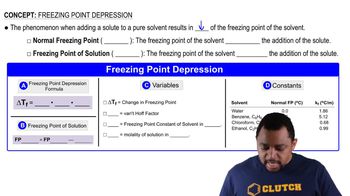

Calculate the freezing point and boiling point of each aqueous solution, assuming complete dissociation of the solute. a. 0.100 m K2S b. 21.5 g of CuCl2 in 4.50⨉102 g water
Calculate the freezing point and boiling point of each aqueous solution, assuming complete dissociation of the solute. c. 5.5% NaNO3 by mass (in water)
Use the van't Hoff factors in Table 13.9 to calculate each colligative property: a. the melting point of a 0.100 m iron(III) chloride solution
A 1.2 m aqueous solution of an ionic compound with the formula MX2 has a boiling point of 101.4 °C. Calculate the van't Hoff factor (i) for MX2 at this concentration.