What mass of salt (NaCl) should you add to 1.00 L of water in an ice cream maker to make a solution that freezes at -10.0 °C? Assume complete dissociation of the NaCl and density of 1.00 g/mL for water.
Ch.14 - Solutions

Chapter 14, Problem 94
A 0.95 m aqueous solution of an ionic compound with the formula MX has a freezing point of -3.0 °C. Calculate the van’t Hoff factor (i) for MX at this concentration.
 Verified step by step guidance
Verified step by step guidance1
insert step 1> Determine the freezing point depression (\( \Delta T_f \)) using the formula \( \Delta T_f = T_f^0 - T_f \), where \( T_f^0 \) is the freezing point of pure water (0 °C) and \( T_f \) is the freezing point of the solution (-3.0 °C).
insert step 2> Use the formula for freezing point depression: \( \Delta T_f = i \cdot K_f \cdot m \), where \( K_f \) is the cryoscopic constant for water (1.86 °C/m) and \( m \) is the molality of the solution (0.95 m).
insert step 3> Rearrange the formula to solve for the van’t Hoff factor \( i \): \( i = \frac{\Delta T_f}{K_f \cdot m} \).
insert step 4> Substitute the known values into the equation: \( i = \frac{3.0}{1.86 \times 0.95} \).
insert step 5> Calculate the value of \( i \) to determine the van’t Hoff factor for the ionic compound MX.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Freezing Point Depression
Freezing point depression is a colligative property that describes how the freezing point of a solvent decreases when a solute is added. The extent of this depression is directly proportional to the number of solute particles in the solution. The formula used to calculate the change in freezing point is ΔTf = i * Kf * m, where ΔTf is the change in freezing point, Kf is the freezing point depression constant, and m is the molality of the solution.
Recommended video:
Guided course

Freezing Point Depression
Van't Hoff Factor (i)
The van't Hoff factor (i) represents the number of particles into which a solute dissociates in solution. For ionic compounds, this factor is crucial as it affects colligative properties like freezing point depression and boiling point elevation. For example, if an ionic compound MX dissociates into M⁺ and X⁻, the van't Hoff factor would be 2, indicating that two particles are produced per formula unit of the solute.
Recommended video:
Guided course
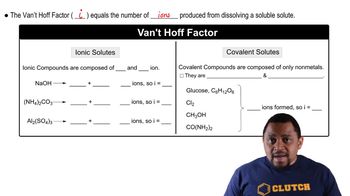
Van't Hoff Factor
Molality (m)
Molality (m) is a measure of concentration defined as the number of moles of solute per kilogram of solvent. It is particularly useful in colligative property calculations because it remains unaffected by temperature changes, unlike molarity. In the context of freezing point depression, knowing the molality of the solution allows for accurate calculations of how much the freezing point will decrease based on the number of solute particles present.
Recommended video:
Guided course
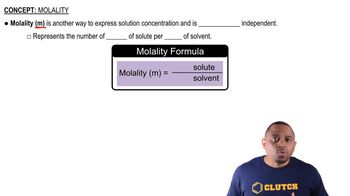
Molality
Related Practice
Textbook Question
1
views
Textbook Question
Use the van't Hoff factors in Table 13.9 to calculate each colligative property: a. the melting point of a 0.100 m iron(III) chloride solution
Textbook Question
A 1.2 m aqueous solution of an ionic compound with the formula MX2 has a boiling point of 101.4 °C. Calculate the van't Hoff factor (i) for MX2 at this concentration.
Textbook Question
A 0.100 M ionic solution has an osmotic pressure of 8.3 atm at 25 °C. Calculate the van't Hoff factor (i) for this solution.
Textbook Question
Calculate the vapor pressure at 25 °C of an aqueous solution that is 5.50% NaCl by mass. (Assume complete dissociation of the solute.)
