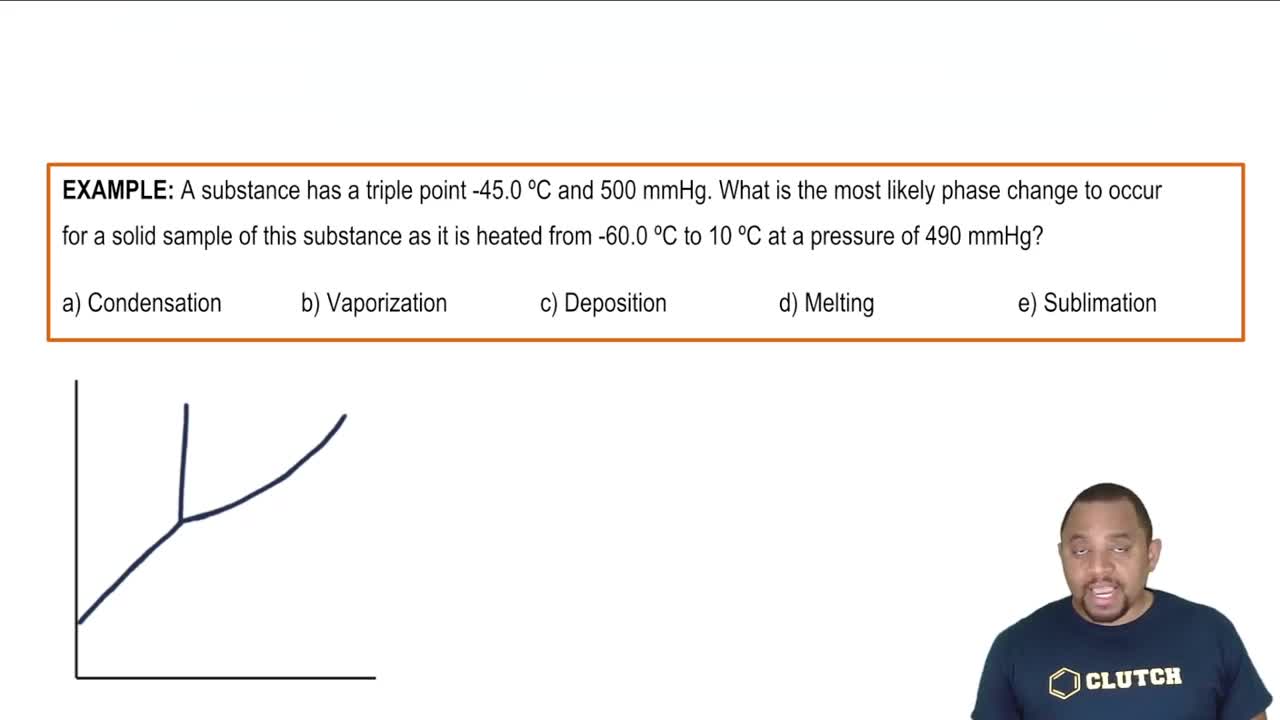
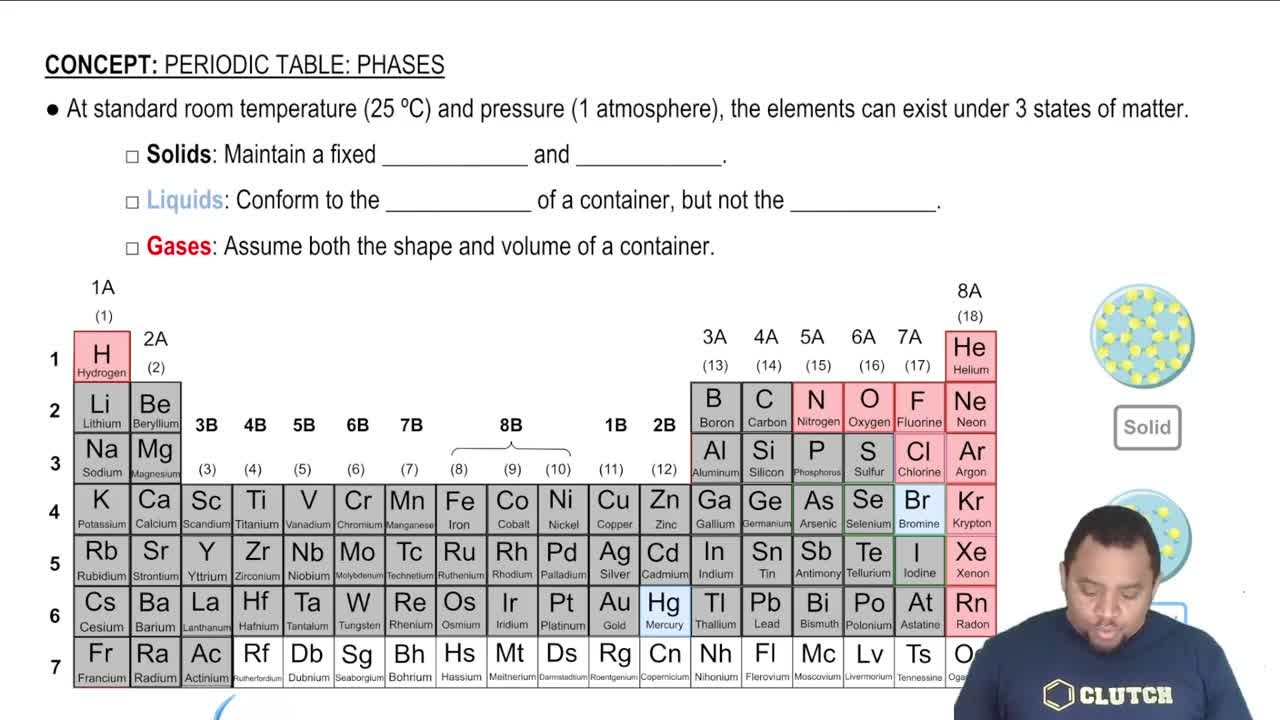
Textbook Question
Mercury is often used in thermometers. The mercury sits in a bulb on the bottom of the thermometer and rises up a thin capillary as the temperature rises. Suppose a mercury thermometer contains 3.380 g of mercury and has a capillary that is 0.200 mm in diameter. How far does the mercury rise in the capillary when the temperature changes from 0.0 °C to 25.0 °C? The density of mercury at these temperatures is 13.596 g/cm3 and 13.534 g/cm3, respectively
4
views