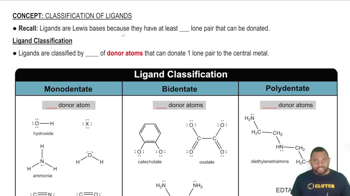
Textbook Question
Balance each chemical equation. b. Co(NO3)3(aq) + (NH4)2S(aq) → Co2S3(s) + NH4NO3(aq)
1
views

 Verified step by step guidance
Verified step by step guidance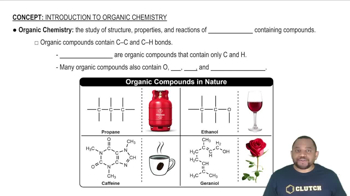

Balance each chemical equation. b. Co(NO3)3(aq) + (NH4)2S(aq) → Co2S3(s) + NH4NO3(aq)
Balance each chemical equation. a. Na2S(aq) + Cu(NO3)2(aq) → NaNO3(aq) + CuS(s) b. N2H4(l) → NH3(g) + N2(g) c. HCl(aq) + O2(g) → H2O(l) + Cl2(g) d. FeS(s) + HCl(aq) → FeCl2(aq) + H2S(g)
Classify each hydrocarbon as an alkane, alkene, or alkyne. a. H2C=CH−CH3 b. H3C−CH2−CH3 c. HC≡C−CH3 d. H3C−CH2−CH2−CH3
Classify each hydrocarbon as an alkane, alkene, or alkyne. a. HC≡CH