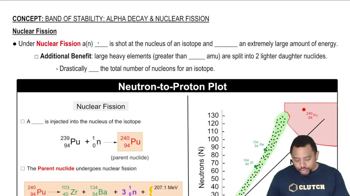
Elemental Composition
The elemental composition, including the number of protons and neutrons, plays a crucial role in determining the isotopes of an element. Elements with higher atomic numbers tend to have more isotopes due to the increased number of neutrons required to offset the repulsive forces between protons. Sodium and aluminum, being lighter elements, have fewer stable isotopes as their nuclear configurations do not support multiple stable forms.

 Verified step by step guidance
Verified step by step guidance