Textbook Question
Write a nuclear equation for the indicated decay of each nuclide. c. Pb-214 (beta)

 Verified step by step guidance
Verified step by step guidance
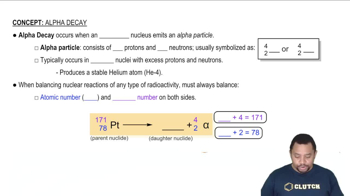
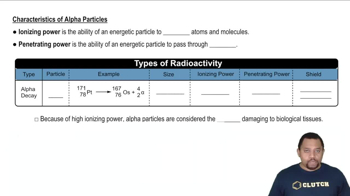
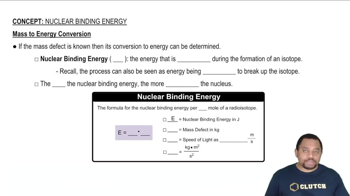
Write a nuclear equation for the indicated decay of each nuclide. c. Pb-214 (beta)
Write a nuclear equation for the indicated decay of each nuclide. d. N-13 (positron emission)
Write a nuclear equation for the indicated decay of each nuclide. e. Cr-51 (electron capture)