A rechargeable battery is constructed based on a concentration cell constructed of two Ag/Ag+ half-cells. The volume of each half-cell is 2.0 L, and the concentrations of Ag+ in the half-cells are 1.25 M and 1.0 × 10–3 M. c. Upon recharging, how long would it take to redissolve 1.00 × 102 g of silver at a charging current of 10.0 amps?
Ch.19 - Electrochemistry

Chapter 19, Problem 119
The Ksp of CuI is 1.1 * 10^-12. Find Ecell for the cell: Cu(s) | CuI(s) | I^-(aq)(1.0 M) || Cu^+(aq)(1.0 M) | Cu(s)
 Verified step by step guidance
Verified step by step guidance1
Identify the half-reactions involved in the cell. The dissolution of CuI involves the equilibrium: CuI(s) ⇌ Cu⁺(aq) + I⁻(aq).
Write the Nernst equation for the cell: E_{cell} = E^0_{cell} - \frac{RT}{nF} \ln Q, where Q is the reaction quotient.
Determine the standard cell potential, E^0_{cell}, using standard reduction potentials: Cu⁺ + e⁻ → Cu(s) and I⁻ → I₂ + 2e⁻.
Calculate the reaction quotient, Q, using the concentrations given: Q = \frac{[Cu^+]}{[I^-]}.
Substitute the values into the Nernst equation to find E_{cell}, considering the temperature is 298 K and n = 1 for the electron transfer.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Solubility Product Constant (Ksp)
The solubility product constant (Ksp) is an equilibrium constant that applies to the solubility of sparingly soluble ionic compounds. It represents the product of the molar concentrations of the ions, each raised to the power of their coefficients in the balanced equation. For CuI, Ksp = [Cu^+][I^-], indicating how much of the compound can dissolve in water before reaching saturation.
Recommended video:
Guided course

Solubility Product Constant
Electrochemical Cell and Ecell
An electrochemical cell consists of two half-cells where oxidation and reduction reactions occur. Ecell, or cell potential, is the measure of the voltage produced by the cell, calculated using the standard reduction potentials of the half-reactions involved. It indicates the driving force for the electrochemical reaction, with positive values suggesting spontaneous reactions.
Recommended video:
Guided course
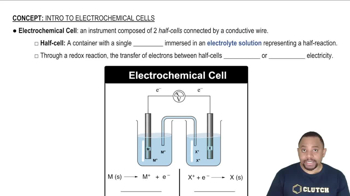
Electrochemical Cells
Nernst Equation
The Nernst equation relates the cell potential (Ecell) to the concentrations of the reactants and products in an electrochemical reaction. It is expressed as Ecell = E°cell - (RT/nF)ln(Q), where E°cell is the standard cell potential, R is the gas constant, T is the temperature in Kelvin, n is the number of moles of electrons transferred, F is Faraday's constant, and Q is the reaction quotient. This equation allows for the calculation of Ecell under non-standard conditions.
Recommended video:
Guided course
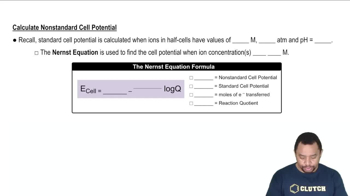
The Nernst Equation
Related Practice
Textbook Question
Textbook Question
Calculate ∆G°rxn and K for each reaction.
a. The disproportionation of Mn2+(aq) to Mn(s) and MnO2(s) in acid solution at 25 °C.
b. The disproportionation of MnO2(s) to Mn2+(aq) and MnO4–(aq) in acid solution at 25 °C.
Textbook Question
Calculate ∆G°rxn and K for each reaction. a. The reaction of Cr2+(aq) with Cr2O72–(aq) in acid solution to form Cr3+(aq).
Textbook Question
Calculate ∆G°rxn and K for each reaction. b. The reaction of Cr3+(aq) and Cr(s) to form Cr2+(aq). [The electrode potential of Cr2+(aq) to Cr(s) is -0.91 V.]
