A battery relies on the oxidation of magnesium and the reduction of Cu2+. The initial concentrations of Mg2+ and Cu2+ are 1.0 × 10–4 M and 1.5 M, respectively, in 1.0-liter half-cells. c. How long can the battery deliver 5.0 A before going dead?
Ch.19 - Electrochemistry

Chapter 19, Problem 116c
A rechargeable battery is constructed based on a concentration cell constructed of two Ag/Ag+ half-cells. The volume of each half-cell is 2.0 L, and the concentrations of Ag+ in the half-cells are 1.25 M and 1.0 × 10–3 M. c. Upon recharging, how long would it take to redissolve 1.00 × 102 g of silver at a charging current of 10.0 amps?
 Verified step by step guidance
Verified step by step guidance1
Calculate the number of moles of silver (Ag) that need to be redissolved using the formula: \( \text{moles of Ag} = \frac{\text{mass of Ag}}{\text{molar mass of Ag}} \). The molar mass of silver is approximately 107.87 g/mol.
Use Faraday's laws of electrolysis to relate the charge required to redissolve the silver. The formula is: \( Q = n \times F \), where \( Q \) is the total charge in coulombs, \( n \) is the number of moles of electrons, and \( F \) is Faraday's constant (approximately 96485 C/mol). Since each Ag atom requires one electron to be reduced, \( n \) is equal to the moles of Ag.
Determine the time required to supply this charge using the formula: \( t = \frac{Q}{I} \), where \( t \) is the time in seconds, \( Q \) is the charge in coulombs, and \( I \) is the current in amperes (10.0 A in this case).
Convert the time from seconds to a more convenient unit if necessary, such as minutes or hours, by using appropriate conversion factors.
Summarize the process: Calculate moles of Ag, determine the charge needed using Faraday's law, and then find the time required using the current provided.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
5mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Concentration Cells
A concentration cell is a type of electrochemical cell where two half-cells have different concentrations of the same ion. The cell generates an electromotive force (EMF) due to the difference in concentration, driving the spontaneous flow of electrons from the higher concentration to the lower concentration. This principle is crucial for understanding how rechargeable batteries operate, particularly in the context of redox reactions.
Recommended video:
Guided course

The Electrolytic Cell
Faraday's Law of Electrolysis
Faraday's Law states that the amount of substance transformed during electrolysis is directly proportional to the quantity of electric charge passed through the cell. This law allows us to calculate the mass of a substance, such as silver, that can be deposited or dissolved during the charging process, using the formula: mass = (current × time × molar mass) / (n × F), where n is the number of moles of electrons transferred and F is Faraday's constant.
Recommended video:
Guided course
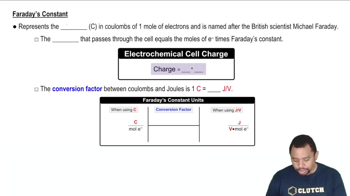
Faraday's Constant in Electrochemistry
Current and Time Relationship
The relationship between current, time, and charge is fundamental in electrochemistry. Current (measured in amperes) is defined as the flow of electric charge per unit time. To determine how long it takes to dissolve a specific mass of silver, one must relate the total charge required (calculated from the mass of silver) to the current applied, using the formula: time = total charge / current.
Recommended video:
Guided course
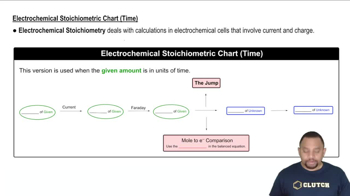
Electrochemical Stoichiometric Chart (Time)
Related Practice
Textbook Question
Textbook Question
A rechargeable battery is constructed based on a concentration cell constructed of two Ag/Ag+ half-cells. The volume of each half-cell is 2.0 L, and the concentrations of Ag+ in the half-cells are 1.25 M and 1.0×10–3 M. a. How long can this battery deliver 2.5 Aof current before it goes dead?
Textbook Question
A rechargeable battery is constructed based on a concentration cell constructed of two Ag/Ag+ half-cells. The volume of each half-cell is 2.0 L, and the concentrations of Ag+ in the half-cells are 1.25 M and 1.0 × 10–3 M. b. What mass of silver is plated onto the cathode by running at 3.5 A for 5.5 h?
