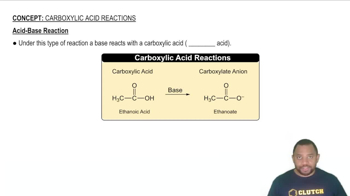
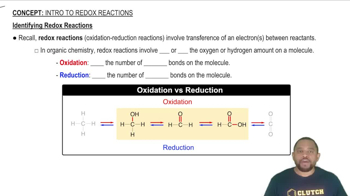
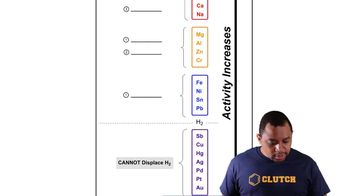
Determine whether or not each redox reaction occurs spontaneously in the forward direction.
a. Ca2+(aq) + Zn(s) → Ca(s) + Zn2+(aq)
b. 2 Ag+(aq) + Ni(s) → 2 Ag(s) + Ni2+(aq)
c. Fe(s) + Mn2+(aq) → Fe2+(aq) + Mn(s)
d. 2 Al(s) + 3 Pb2+(aq) → 2 Al3+(aq) + 3 Pb(s)