Without doing any calculations, determine the sign of ΔSsys for each chemical reaction. a. 2 KClO3(s) → 2 KCl(s) + 3 O2(g) c. Na(s) + 2 Cl2(g) → NaCl(s) d. N2(g) + 3 H2(g) → 2 NH3(g)
Ch.18 - Free Energy and Thermodynamics

Chapter 18, Problem 36
Without doing any calculations, determine the sign of ΔSsys for each chemical reaction. a. Mg(s) + Cl2(g) → MgCl2(s) b. 2 H2S(g) + 3 O2(g) → 2 H2O(g) + 2 SO2(g) c. 2 O3(g) → 3 O2(g) d. HCl(g) + NH3(g) → NH4Cl(s)
 Verified step by step guidance
Verified step by step guidance1
Identify the states of matter for each reactant and product in the reactions.
Recall that entropy (ΔS) is a measure of disorder or randomness in a system, with gases having higher entropy than liquids or solids.
For reaction (a), note that a gas and a solid react to form a solid, indicating a decrease in entropy (ΔSsys < 0).
For reaction (b), compare the number of gas molecules on both sides of the equation. The number of gas molecules remains the same, suggesting a small change in entropy (ΔSsys ≈ 0).
For reaction (c), observe that the reaction goes from 2 moles of gas to 3 moles of gas, indicating an increase in entropy (ΔSsys > 0).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Entropy (ΔS)
Entropy, denoted as ΔS, is a measure of the disorder or randomness in a system. In chemical reactions, changes in entropy can indicate whether the products are more or less disordered than the reactants. A positive ΔS suggests an increase in disorder, while a negative ΔS indicates a decrease in disorder.
Recommended video:
Guided course
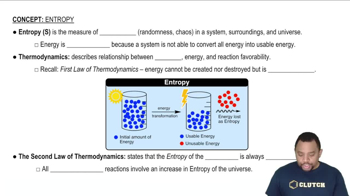
Entropy in Thermodynamics
Phase Changes and Entropy
The phase of a substance significantly affects its entropy. Gases have higher entropy than liquids, which in turn have higher entropy than solids due to the greater freedom of movement and arrangement of particles in gases. Therefore, reactions that produce gases from solids or liquids typically result in a positive ΔS.
Recommended video:
Guided course
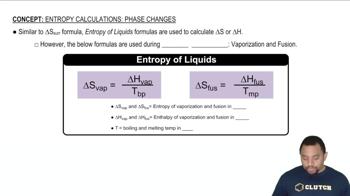
Entropy in Phase Changes
Molecular Complexity and Entropy
The complexity and number of molecules in a reaction can influence entropy. Reactions that produce more molecules or more complex molecules generally lead to an increase in entropy. Conversely, reactions that result in fewer or simpler molecules tend to decrease the system's entropy.
Recommended video:
Guided course
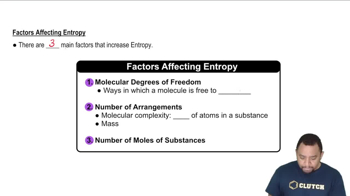
Factors Affecting Entropy
Related Practice
Textbook Question
1
views
Textbook Question
Without doing any calculations, determine the sign of ΔSsys for each chemical reaction. b. CH2=CH2( g) + H2( g) → CH3CH3( g)
Textbook Question
Without doing any calculations, determine the signs of ΔSsys and ΔS surr for each chemical reaction. In addition, predict under what temperatures (all temperatures, low temperatures, or high temperatures), if any, the reaction is spontaneous. a. C3H8(g) + 5 O2(g) → 3 CO2(g) + 4 H2O(g) ΔH°rxn = -2044 kJ
1
views
Textbook Question
Without doing any calculations, determine the signs of ΔSsys and ΔSsurr for each chemical reaction. In addition, predict under what temperatures (all temperatures, low temperatures, or high temperatures), if any, the reaction is spontaneous. c. 2 N2(g) + O2(g) → 2 N2O(g) ΔH°rxn = +163.2 kJ
