Textbook Question
A reaction has an equilibrium constant of 8.5⨉103 at 298 K. At 755 K, the equilibrium constant is 0.65. Find ΔH°rxn for the reaction.

 Verified step by step guidance
Verified step by step guidance

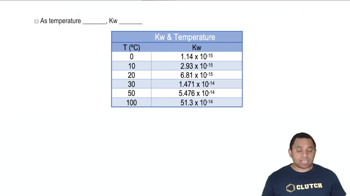
A reaction has an equilibrium constant of 8.5⨉103 at 298 K. At 755 K, the equilibrium constant is 0.65. Find ΔH°rxn for the reaction.
Determine the sign of ΔSsys for each process. a. water boiling
Determine the sign of ΔSsys for each process. b. water freezing
Nitrogen dioxide, a pollutant in the atmosphere, can combine with water to form nitric acid. One of the possible reactions is shown here. Calculate ΔG° and Kp for this reaction at 25 °C and comment on the spontaneity of the reaction. 3 NO2(g) + H2O(l)→ 2 HNO3(aq) + NO(g)