Nitrogen dioxide, a pollutant in the atmosphere, can combine with water to form nitric acid. One of the possible reactions is shown here. Calculate ΔG° and Kp for this reaction at 25 °C and comment on the spontaneity of the reaction. 3 NO2(g) + H2O(l)→ 2 HNO3(aq) + NO(g)
Ch.18 - Free Energy and Thermodynamics

Chapter 18, Problem 88
H2 reacts with the halogens (X2) according to the reaction: H2(g) + X2(g) → 2 HX(g) where X2 can be Cl2, Br2, or I2. Use the thermodynamic data in Appendix IIB to calculate ΔH°, ΔS°, ΔG°, and Kp for the reaction between hydrogen and each of the three halogens. Which reaction is most spontaneous? Least spontaneous? What is the main factor responsible for the difference in the spontaneity of the three reactions? Does higher temperature make the reactions more spontaneous or less spontaneous?
 Verified step by step guidance
Verified step by step guidance1
Identify the thermodynamic data needed for each reaction: \( \Delta H^\circ_f \), \( \Delta S^\circ \), and \( \Delta G^\circ_f \) for each reactant and product from Appendix IIB.
Calculate \( \Delta H^\circ \) for each reaction using the formula: \( \Delta H^\circ = \sum \Delta H^\circ_f(\text{products}) - \sum \Delta H^\circ_f(\text{reactants}) \).
Calculate \( \Delta S^\circ \) for each reaction using the formula: \( \Delta S^\circ = \sum \Delta S^\circ(\text{products}) - \sum \Delta S^\circ(\text{reactants}) \).
Calculate \( \Delta G^\circ \) for each reaction using the formula: \( \Delta G^\circ = \Delta H^\circ - T\Delta S^\circ \), where \( T \) is the temperature in Kelvin.
Determine \( K_p \) for each reaction using the relationship: \( \Delta G^\circ = -RT \ln K_p \), and compare the spontaneity of the reactions by analyzing \( \Delta G^\circ \) and \( K_p \).
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Thermodynamic Functions
Thermodynamic functions such as enthalpy (ΔH°), entropy (ΔS°), and Gibbs free energy (ΔG°) are essential for understanding chemical reactions. ΔH° indicates the heat absorbed or released during a reaction, while ΔS° measures the change in disorder. ΔG° combines these two to determine the spontaneity of a reaction; a negative ΔG° indicates a spontaneous process. Understanding these functions helps predict how reactions will behave under different conditions.
Recommended video:
Guided course

First Law of Thermodynamics
Equilibrium Constant (Kp)
The equilibrium constant (Kp) quantifies the ratio of the concentrations of products to reactants at equilibrium for a given reaction. For the reaction H2 + X2 → 2HX, Kp can be calculated using the partial pressures of the gases involved. A larger Kp value indicates a greater tendency for the reaction to favor product formation, which is closely related to the spontaneity of the reaction. Understanding Kp is crucial for analyzing how changes in conditions affect reaction outcomes.
Recommended video:
Guided course

Equilibrium Constant Expressions
Effect of Temperature on Spontaneity
Temperature plays a significant role in the spontaneity of chemical reactions, as described by the Gibbs free energy equation: ΔG° = ΔH° - TΔS°. An increase in temperature can enhance the spontaneity of reactions with positive ΔS° (increased disorder) by reducing ΔG°. Conversely, for reactions with negative ΔS°, higher temperatures may make them less spontaneous. Thus, analyzing the temperature's effect is vital for understanding the behavior of the reactions between hydrogen and halogens.
Recommended video:
Guided course
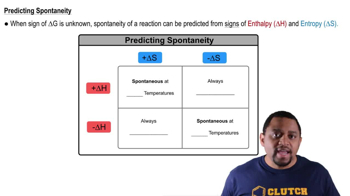
Spontaneity and Temperature
Related Practice
Textbook Question
Textbook Question
Consider this reaction occurring at 298 K: N2O(g) + NO2(g) ⇌ 3 NO(g) a. Show that the reaction is not spontaneous under standard conditions by calculating ΔG°rxn.
Textbook Question
Consider this reaction occurring at 298 K: N2O(g) + NO2(g) ⇌ 3 NO(g) b. If a reaction mixture contains only N2O and NO2 at partial pressures of 1.0 atm each, the reaction will be spontaneous until some NO forms in the mixture. What maximum partial pressure of NO builds up before the reaction ceases to be spontaneous?
Textbook Question
Consider this reaction occurring at 298 K: N2O(g) + NO2(g) ⇌ 3 NO(g) c. Can the reaction be made more spontaneous by an increase or decrease in temperature? If so, what temperature is required to make the reaction spontaneous under standard conditions?
