In each reaction, identify the Brønsted–Lowry acid, the Brønsted–Lowry base, the conjugate acid, and the conjugate base. a. H2CO3(aq) + H2O(l) ⇌ H3O+(aq) + HCO3–(aq) c. HNO3(aq) + H2O(l) → H3O+(aq) + NO3–(aq)
Ch.16 - Acids and Bases

Chapter 16, Problem 36b
In each reaction, identify the Brønsted–Lowry acid, the Brønsted–Lowry base, the conjugate acid, and the conjugate base. b. CH3NH2(aq) + H2O(l) ⇌ CH3NH3+(aq) + OH–(aq)
 Verified step by step guidance
Verified step by step guidance1
Identify the Brønsted–Lowry acid and base in the reactants: The Brønsted–Lowry acid is the species that donates a proton (H+), and the Brønsted–Lowry base is the species that accepts a proton.
In the reaction CH3NH2(aq) + H2O(l) -> CH3NH3+(aq) + OH-(aq), identify which species donates a proton and which accepts it.
CH3NH2 is the Brønsted–Lowry base because it accepts a proton from H2O, forming CH3NH3+.
H2O is the Brønsted–Lowry acid because it donates a proton to CH3NH2, forming OH-.
Identify the conjugate acid and conjugate base: The conjugate acid is the species formed when the base gains a proton, and the conjugate base is the species formed when the acid loses a proton. CH3NH3+ is the conjugate acid, and OH- is the conjugate base.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Brønsted–Lowry Acid-Base Theory
The Brønsted–Lowry theory defines acids as proton donors and bases as proton acceptors. In this framework, an acid-base reaction involves the transfer of protons (H+) from the acid to the base. This theory expands the understanding of acid-base reactions beyond just the presence of hydroxide ions, allowing for a broader range of substances to be classified as acids or bases.
Recommended video:
Guided course

Bronsted-Lowry Acid-Base Theory
Conjugate Acid and Conjugate Base
In the context of Brønsted–Lowry theory, a conjugate acid is formed when a base accepts a proton, while a conjugate base is formed when an acid donates a proton. These pairs are related; for every acid, there is a corresponding conjugate base, and for every base, there is a corresponding conjugate acid. Understanding these relationships is crucial for analyzing acid-base reactions.
Recommended video:
Guided course
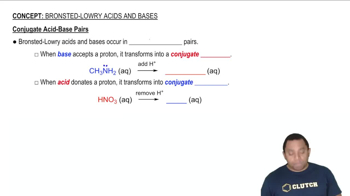
Conjugate Acid-Base Pairs
Identifying Species in Reactions
To analyze acid-base reactions, it is essential to identify the reactants and products involved. In the given reaction, CH3NH2 acts as a base by accepting a proton from water (H2O), which acts as an acid. The products, CH3NH3+ and OH-, represent the conjugate acid and conjugate base, respectively. Recognizing these roles is key to understanding the dynamics of the reaction.
Recommended video:
Guided course
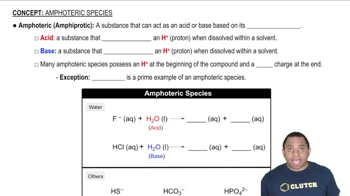
Amphoteric Species
Related Practice
Textbook Question
Textbook Question
In each reaction, identify the Brønsted–Lowry acid, the Brønsted–Lowry base, the conjugate acid, and the conjugate base. b. NH3(aq) + H2O(l) ⇌ NH4+(aq) + OH–(aq)
Textbook Question
In each reaction, identify the Brønsted–Lowry acid, the Brønsted–Lowry base, the conjugate acid, and the conjugate base. d. C5H5N(aq) + H2O(l) ⇌ C5H5NH+(aq) + OH–(aq)
Textbook Question
In each reaction, identify the Brønsted–Lowry acid, the Brønsted–Lowry base, the conjugate acid, and the conjugate base. c. CO32–(aq) + H2O(l) ⇌ HCO3–(aq) + OH–(aq)
Textbook Question
Write the formula for the conjugate base of each acid. a. HCl
Textbook Question
Write the formula for the conjugate base of each acid. b. H2SO3
