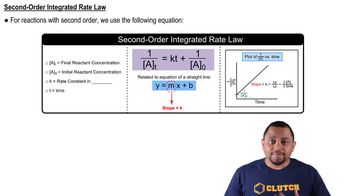
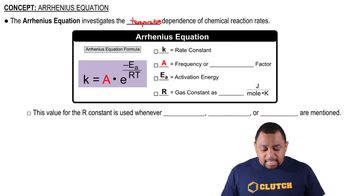
The data shown here were collected for the first-order reaction: N2O(g) → N2(g) + O(g) Use an Arrhenius plot to determine the activation barrier and frequency factor for the reaction.
Temperature (K) Rate Constant (1 , s)
800 3.24⨉10- 5
900 0.00214
1000 0.0614
1100 0.955