The rate constant (k) for a reaction was measured as a function of temperature. A plot of ln k versus 1/T (in K) is linear and has a slope of -7445 K. Calculate the activation energy for the reaction.
Ch.14 - Chemical Kinetics

Chapter 14, Problem 65
The data shown here were collected for the first-order reaction: N2O(g) → N2(g) + O(g) Use an Arrhenius plot to determine the activation barrier and frequency factor for the reaction.
Temperature (K) Rate Constant (1 , s)
800 3.24⨉10- 5
900 0.00214
1000 0.0614
1100 0.955
 Verified step by step guidance
Verified step by step guidance1
First, understand that an Arrhenius plot graphs the natural logarithm of the rate constant (ln(k)) against the reciprocal of the temperature in Kelvin (1/T). This plot helps determine the activation energy and the frequency factor of a reaction.
Next, convert the given temperatures into their reciprocal form (1/T) where T is the temperature in Kelvin. This will be the x-axis of your Arrhenius plot.
Then, take the natural logarithm of each rate constant (ln(k)). These values will form the y-axis of your Arrhenius plot.
Plot the points with the reciprocal of the temperature on the x-axis and the natural logarithm of the rate constant on the y-axis. Draw the best fit line through these points.
Finally, the slope of the line from the Arrhenius plot will give you the negative activation energy divided by the gas constant (R), and the y-intercept will give you the natural logarithm of the frequency factor (ln(A)).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
First-Order Reactions
First-order reactions are chemical reactions where the rate is directly proportional to the concentration of one reactant. This means that if the concentration of the reactant doubles, the reaction rate also doubles. Understanding this concept is crucial for analyzing the provided rate constants at different temperatures, as it allows for the application of the integrated rate law to determine the relationship between concentration and time.
Recommended video:
Guided course

First-Order Reactions
Arrhenius Equation
The Arrhenius equation describes how the rate constant of a reaction depends on temperature and activation energy. It is expressed as k = A * e^(-Ea/RT), where k is the rate constant, A is the frequency factor, Ea is the activation energy, R is the gas constant, and T is the temperature in Kelvin. This equation is fundamental for constructing an Arrhenius plot, which is a graph of ln(k) versus 1/T, allowing for the determination of both the activation energy and frequency factor from the slope and intercept.
Recommended video:
Guided course
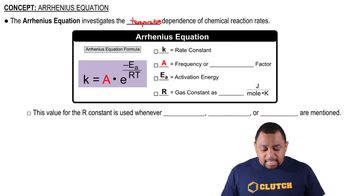
Arrhenius Equation
Activation Energy
Activation energy (Ea) is the minimum energy required for a chemical reaction to occur. It represents the energy barrier that reactants must overcome to transform into products. In the context of the Arrhenius equation, a higher activation energy results in a lower rate constant at a given temperature, indicating that the reaction is slower. Understanding activation energy is essential for interpreting the results from the Arrhenius plot and assessing the feasibility of the reaction under different thermal conditions.
Recommended video:
Guided course

Activity Series Chart
Related Practice
Textbook Question
1
views
Textbook Question
The tabulated data show the rate constant of a reaction measured at several different temperatures. Use an Arrhenius plot to determine the activation barrier and frequency factor for the reaction.
Temperature (K) Rate Constant (1 , s)
300 0.0134
310 0.0407
320 0.114
330 0.303
340 0.757
Textbook Question
The tabulated data show the rate constant of a reaction measured at several different temperatures. Use an Arrhenius plot to determine the activation barrier and frequency factor for the reaction.
Temperature (K) Rate Constant (1 , s)
310 0.00434
320 0.0140
330 0.0421
340 0.118
350 0.316
