Textbook Question
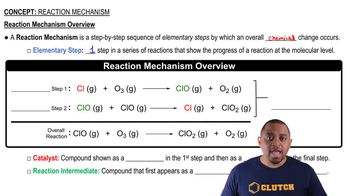
Consider this three-step mechanism for a reaction:
Cl2 (g) k1⇌k2 2 Cl (g) Fast
Cl (g) + CHCl3 (g) →k3 HCl (g) + CCl3 (g) Slow
Cl (g) + CCl3 (g) →k4 CCl4 (g) Fast
a. What is the overall reaction?

 Verified step by step guidance
Verified step by step guidance

Consider this three-step mechanism for a reaction:
Cl2 (g) k1⇌k2 2 Cl (g) Fast
Cl (g) + CHCl3 (g) →k3 HCl (g) + CCl3 (g) Slow
Cl (g) + CCl3 (g) →k4 CCl4 (g) Fast
a. What is the overall reaction?
Consider this three-step mechanism for a reaction:
Cl2 (g) k1⇌k2 2 Cl (g) Fast
Cl (g) + CHCl3 (g) →k3 HCl (g) + CCl3 (g) Slow
Cl (g) + CCl3 (g) →k4 CCl4 (g) Fast
b. Identify the intermediates in the mechanism.
Consider this two-step mechanism for a reaction: NO2(g) + Cl2(g) → k1 ClNO2(g) + Cl g) Slow NO2(g) + Cl(g) →k2 ClNO2(g) Fast b. Identify the intermediates in the mechanism.
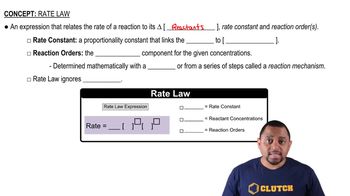
Many heterogeneous catalysts are deposited on high-surfacearea supports. Why?