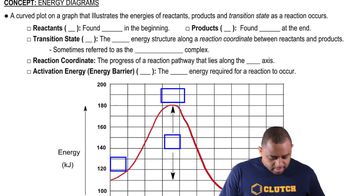
Energy Diagram
An energy diagram visually represents the energy changes that occur during a chemical reaction. It typically shows the energy of reactants, products, and the transition states, indicating the activation energy required for the reaction to proceed. By examining the diagram, one can identify the number of steps and the energy barriers involved in the reaction.

 Verified step by step guidance
Verified step by step guidance