A reaction in which A, B, and C react to form products is zero order in A, one-half order in B, and second order in C. c. By what factor does the reaction rate change if [A] is doubled (and the other reactant concentrations are held constant)? d. By what factor does the reaction rate change if [B] is doubled? e. By what factor does the reaction rate change if [C] is doubled? f. By what factor does the reaction rate change if [C] is doubled (and the other reactant concentrations are held constant)?
Ch.14 - Chemical Kinetics

Chapter 14, Problem 43
Consider the tabulated data showing the initial rate of a reaction (A → products) at several different concentrations of A. What is the order of the reaction? Write a rate law for the reaction including the value of the rate constant, k.
 Verified step by step guidance
Verified step by step guidance1
Identify the general form of the rate law for the reaction: \( \text{Rate} = k[A]^n \), where \( n \) is the order of the reaction.
Examine the tabulated data to determine how the initial rate changes with varying concentrations of \( A \).
Compare the rates and concentrations to find a pattern: if doubling \([A]\) doubles the rate, the reaction is first order; if doubling \([A]\) quadruples the rate, it is second order, etc.
Use the determined order \( n \) to write the rate law: \( \text{Rate} = k[A]^n \).
Calculate the rate constant \( k \) using one set of data from the table by substituting the values of rate and \([A]\) into the rate law equation.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Reaction Order
The order of a reaction refers to the power to which the concentration of a reactant is raised in the rate law. It indicates how the rate of reaction is affected by the concentration of reactants. For example, if the rate doubles when the concentration of A is doubled, the reaction is first order with respect to A. Understanding reaction order is crucial for determining the rate law.
Recommended video:
Guided course

Average Bond Order
Rate Law
The rate law expresses the relationship between the rate of a chemical reaction and the concentrations of its reactants. It is typically written in the form Rate = k[A]^n, where k is the rate constant and n is the order of the reaction with respect to reactant A. The rate law can be determined experimentally and provides insight into the mechanism of the reaction.
Recommended video:
Guided course
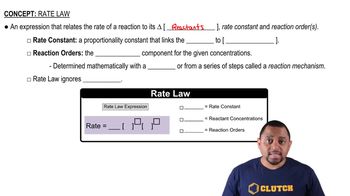
Rate Law Fundamentals
Rate Constant (k)
The rate constant, k, is a proportionality factor in the rate law that is specific to a particular reaction at a given temperature. It reflects the speed of the reaction and varies with temperature and the presence of catalysts. The value of k can be determined from experimental data and is essential for calculating the rate of reaction under different conditions.
Recommended video:
Guided course

Equilibrium Constant K
Related Practice
Textbook Question
1
views
Textbook Question
Consider the tabulated data showing the initial rate of a reaction (A → products) at several different concentrations of A. What is the order of the reaction? Write a rate law for the reaction including the value of the rate constant, k.
Textbook Question
The tabulated data were collected for this reaction: CH3Cl(g) + 3 Cl2(g) → CCl4( g) + 3 HCl(g)
Write an expression for the reaction rate law and calculate the value of the rate constant, k. What is the overall order of the reaction?
