A reaction in which A, B, and C react to form products is zero order in A, one-half order in B, and second order in C. a. Write a rate law for the reaction.
Ch.14 - Chemical Kinetics

Chapter 14, Problem 41
Consider the data showing the initial rate of a reaction (A → products) at several different concentrations of A. What is the order of the reaction? Write a rate law for the reaction including the value of the rate constant, k.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the rate law equation. The rate law for a reaction A → products is generally expressed as rate = k[A]^n, where k is the rate constant, [A] is the concentration of reactant A, and n is the order of the reaction with respect to A.
Step 2: Analyze the data provided. Look at how the initial rate of the reaction changes as the concentration of A changes. This will help determine the order of the reaction.
Step 3: Determine the reaction order. If the rate doubles when [A] doubles, the reaction is first order (n=1). If the rate quadruples when [A] doubles, the reaction is second order (n=2). If the rate remains unchanged when [A] changes, the reaction is zero order (n=0).
Step 4: Write the rate law. Once the order n is determined, write the rate law as rate = k[A]^n.
Step 5: Calculate the rate constant k. Use one set of data (concentration and rate) to solve for k in the rate law equation.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Reaction Order
The order of a reaction refers to the power to which the concentration of a reactant is raised in the rate law. It indicates how the rate of reaction is affected by the concentration of reactants. For example, a first-order reaction means that doubling the concentration of the reactant will double the rate of reaction, while a second-order reaction means that doubling the concentration will quadruple the rate.
Recommended video:
Guided course

Average Bond Order
Rate Law
The rate law expresses the relationship between the rate of a chemical reaction and the concentrations of its reactants. It is typically written in the form Rate = k[A]^n, where k is the rate constant, [A] is the concentration of reactant A, and n is the order of the reaction with respect to A. The rate law can be determined experimentally and provides insight into the mechanism of the reaction.
Recommended video:
Guided course
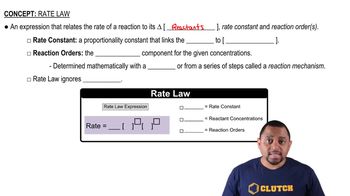
Rate Law Fundamentals
Rate Constant (k)
The rate constant, k, is a proportionality factor in the rate law that is specific to a particular reaction at a given temperature. It reflects the speed of the reaction; a larger k indicates a faster reaction. The value of k can be determined from experimental data and is influenced by factors such as temperature and the presence of catalysts.
Recommended video:
Guided course

Equilibrium Constant K
Related Practice
Textbook Question
Textbook Question
A reaction in which A, B, and C react to form products is zero order in A, one-half order in B, and second order in C. c. By what factor does the reaction rate change if [A] is doubled (and the other reactant concentrations are held constant)? d. By what factor does the reaction rate change if [B] is doubled? e. By what factor does the reaction rate change if [C] is doubled? f. By what factor does the reaction rate change if [C] is doubled (and the other reactant concentrations are held constant)?
1
views
Textbook Question
Consider the tabulated data showing the initial rate of a reaction (A → products) at several different concentrations of A. What is the order of the reaction? Write a rate law for the reaction including the value of the rate constant, k.
