Consider the reaction: C4H8( g) → 2 C2H4( g) The tabulated data were collected for the concentration of C2H4 as a function of time: a. What is the average rate of the reaction between 0 and 10 s? Between 40 and 50 s?
Ch.14 - Chemical Kinetics

Chapter 14, Problem 32b
Consider the reaction: NO2(g) → NO(g) + 1/2 O2( g) The tabulated data were collected for the concentration of NO2 as a function of time: b. What is the rate of formation of O2 between 50 and 60 s?
 Verified step by step guidance
Verified step by step guidance1
Determine the rate of disappearance of NO_2 by calculating the change in concentration of NO_2 over the change in time between 50 and 60 seconds.
Use the stoichiometry of the reaction to relate the rate of disappearance of NO_2 to the rate of formation of O_2. Since 1 mole of O_2 is formed for every 2 moles of NO_2 that decompose, the rate of formation of O_2 is half the rate of disappearance of NO_2.
Express the rate of formation of O_2 in terms of the rate of disappearance of NO_2 using the relationship: Rate of formation of O_2 = (1/2) * Rate of disappearance of NO_2.
Substitute the calculated rate of disappearance of NO_2 into the expression to find the rate of formation of O_2.
Ensure the units are consistent, typically moles per liter per second (M/s), and interpret the result in the context of the reaction.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Reaction Rate
The reaction rate is a measure of how quickly reactants are converted into products in a chemical reaction. It can be expressed as the change in concentration of a reactant or product over time. In this case, the rate of formation of O2 can be calculated by determining the change in its concentration over the specified time interval.
Recommended video:
Guided course
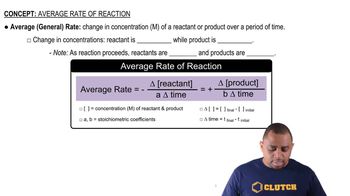
Average Rate of Reaction
Stoichiometry
Stoichiometry involves the quantitative relationships between the reactants and products in a chemical reaction, based on the balanced chemical equation. For the reaction given, the stoichiometric coefficients indicate that for every mole of NO2 that decomposes, half a mole of O2 is produced. This relationship is crucial for calculating the rate of formation of O2 from the change in NO2 concentration.
Recommended video:
Guided course

Stoichiometry Concept
Concentration Change
Concentration change refers to the difference in the amount of a substance present in a given volume over a specific time period. To find the rate of formation of O2, one must first determine the change in concentration of NO2 between 50 and 60 seconds, and then apply stoichiometry to relate this change to the formation of O2.
Recommended video:
Guided course
Chemical Changes
Related Practice
Textbook Question
Textbook Question
Consider the reaction: NO2(g) → NO(g) + 1/2 O2(g) The tabulated data were collected for the concentration of NO2 as a function of time: a. What is the average rate of the reaction between 10 and 20 s? Between 50 and 60 s?
Textbook Question
Consider the reaction: H2(g) + Br2(g) → 2 HBr(g) The graph shows the concentration of Br2 as a function of time.
a. Use the graph to calculate each quantity: (i) the average rate of the reaction between 0 and 25 s
Textbook Question
Consider the reaction: H2(g) + Br2(g) → 2 HBr(g) The graph shows the concentration of Br2 as a function of time. a. Use the graph to calculate each quantity: (iii) the instantaneous rate of formation of HBr at 50 s
