Pick an appropriate solvent from Table 13.3 to dissolve each substance. State the kind of intermolecular forces that would occur between the solute and solvent in each case. a. motor oil (nonpolar) b. ethanol (polar, contains an OH group) c. lard (nonpolar) d. potassium chloride (ionic)

Which molecule would you expect to be more soluble in water: CH3CH2CH2OH or HOCH2CH2CH2OH?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
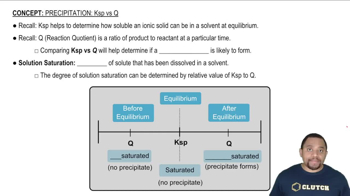
Key Concepts
Polarity of Molecules

Hydrogen Bonding

Hydrophobic vs. Hydrophilic Interactions
Pick an appropriate solvent from Table 13.3 to dissolve each substance. State the kind of intermolecular forces that would occur between the solute and solvent in each case. a. isopropyl alcohol (polar, contains an OH group) c. vegetable oil (nonpolar) d. sodium nitrate (ionic)
Pick an appropriate solvent from Table 13.3 to dissolve each substance. State the kind of intermolecular forces that would occur between the solute and solvent in each case. b. sodium chloride (ionic)
For each compound, would you expect greater solubility in water or in hexane? Indicate the kinds of intermolecular forces that occur between the solute and the solvent in which the molecule is most soluble. a. glucose
For each compound, would you expect greater solubility in water or in hexane? Indicate the kinds of intermolecular forces that would occur between the solute and the solvent in which the molecule is most soluble. d. ethylene glycol
