For each compound, would you expect greater solubility in water or in hexane? Indicate the kinds of intermolecular forces that occur between the solute and the solvent in which the molecule is most soluble. a. glucose
Ch.13 - Solutions

Chapter 13, Problem 35
When ammonium chloride (NH4Cl) is dissolved in water, the solution becomes colder: a. Is the dissolution of ammonium chloride endothermic or exothermic? b. What can you conclude about the relative magnitudes of the lattice energy of ammonium chloride and its heat of hydration? c. Sketch a qualitative energy diagram similar to Figure 13.7 for the dissolution of NH4Cl. d. Why does the solution form? What drives the process?
 Verified step by step guidance
Verified step by step guidance1
Identify the type of reaction: Since the solution becomes colder, the dissolution process absorbs heat from the surroundings, indicating that it is endothermic.
Compare lattice energy and heat of hydration: In an endothermic dissolution, the lattice energy (energy required to break the ionic bonds in the solid) is greater than the heat of hydration (energy released when ions interact with water).
Sketch an energy diagram: Draw an energy diagram with the initial energy of solid NH4Cl higher than the final energy of the dissolved ions, showing an upward arrow to represent the absorption of energy.
Explain solution formation: The solution forms because the increase in entropy (disorder) from dissolving the solid compensates for the energy absorbed, making the process favorable.
Identify the driving force: The process is driven by the increase in entropy, which is a key factor in the spontaneity of the dissolution despite being endothermic.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Endothermic and Exothermic Processes
An endothermic process absorbs heat from the surroundings, resulting in a decrease in temperature, while an exothermic process releases heat, causing an increase in temperature. In the context of ammonium chloride dissolving in water, the solution becoming colder indicates that the dissolution is endothermic, as it requires energy input to break the ionic bonds in the solid.
Recommended video:
Guided course

Endothermic & Exothermic Reactions Example 2
Lattice Energy vs. Heat of Hydration
Lattice energy is the energy required to separate one mole of a solid ionic compound into its gaseous ions, while the heat of hydration is the energy released when these gaseous ions are surrounded by water molecules. For ammonium chloride, if the dissolution is endothermic, it suggests that the lattice energy is greater than the heat of hydration, meaning more energy is needed to break the ionic bonds than is released when the ions interact with water.
Recommended video:
Guided course

Lattice Energy
Energy Diagrams in Chemical Processes
Energy diagrams visually represent the energy changes during a chemical process, illustrating the relative energies of reactants, products, and transition states. For the dissolution of NH4Cl, the diagram would show the initial energy of the solid, a peak representing the energy required to overcome lattice energy, and a lower energy level for the hydrated ions, indicating that the process is driven by the overall energy balance despite the initial energy input.
Recommended video:
Guided course
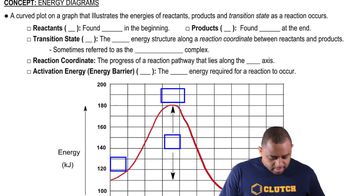
Energy Diagrams
Related Practice
Textbook Question
1
views
1
rank
Textbook Question
For each compound, would you expect greater solubility in water or in hexane? Indicate the kinds of intermolecular forces that would occur between the solute and the solvent in which the molecule is most soluble. d. ethylene glycol
Textbook Question
When lithium iodide (LiI) is dissolved in water, the solution becomes hotter. a. Is the dissolution of lithium iodide endothermic or exothermic?
Textbook Question
When lithium iodide (LiI) is dissolved in water, the solution becomes hotter. b. What can you conclude about the relative magnitudes of the lattice energy of lithium iodide and its heat of hydration?
Textbook Question
When lithium iodide (LiI) is dissolved in water, the solution becomes hotter. c. Sketch a qualitative energy diagram similar to Figure 13.7 for the dissolution of LiI.
