When lithium iodide (LiI) is dissolved in water, the solution becomes hotter. a. Is the dissolution of lithium iodide endothermic or exothermic?
Ch.13 - Solutions

Chapter 13, Problem 36c
When lithium iodide (LiI) is dissolved in water, the solution becomes hotter. c. Sketch a qualitative energy diagram similar to Figure 13.7 for the dissolution of LiI.
 Verified step by step guidance
Verified step by step guidance1
insert step 1> Identify the process: The dissolution of lithium iodide (LiI) in water is an exothermic process, as indicated by the solution becoming hotter.
insert step 2> Understand the energy changes: In an exothermic process, the energy released when new interactions are formed (between Li+ and I- ions and water molecules) is greater than the energy required to break the ionic bonds in LiI and the hydrogen bonds in water.
insert step 3> Sketch the energy diagram: Start with a horizontal line representing the initial energy level of the solid LiI and water.
insert step 4> Show the energy change: Draw a downward arrow from the initial energy level to a lower energy level, representing the energy released during the dissolution process.
insert step 5> Label the diagram: Indicate the initial state (LiI solid + water), the final state (Li+ and I- ions in solution), and the energy released (exothermic) on the diagram.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Dissolution Process
The dissolution process involves the breaking of ionic bonds in a solid solute and the formation of interactions between the solute ions and solvent molecules. In the case of lithium iodide (LiI), the ionic bonds between lithium and iodide ions are disrupted when the solid is added to water, allowing the ions to disperse throughout the solvent.
Recommended video:
Guided course

Spontaneity of Processes
Exothermic Reactions
An exothermic reaction is a process that releases heat to its surroundings. When LiI dissolves in water and the solution becomes hotter, it indicates that the energy released from the formation of ion-dipole interactions between the Li+ and I- ions and water molecules is greater than the energy required to break the ionic bonds in the solid.
Recommended video:
Guided course
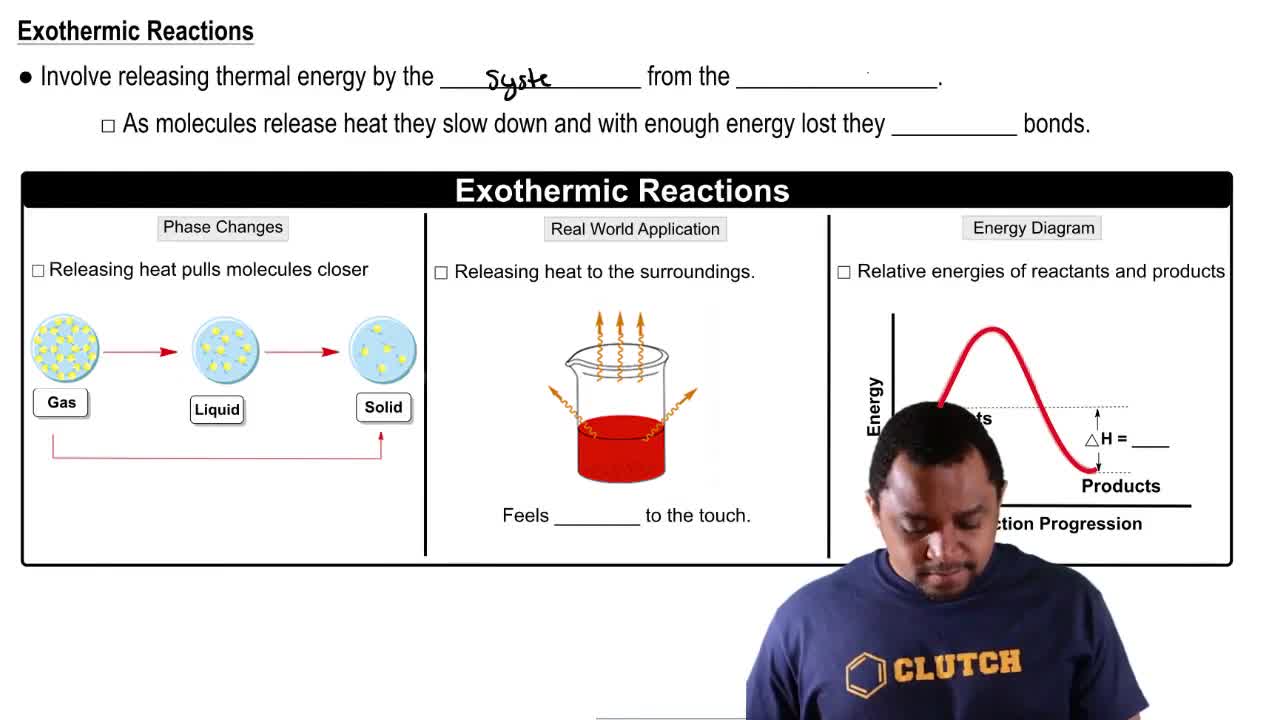
Endothermic & Exothermic Reactions
Energy Diagrams
Energy diagrams visually represent the energy changes during a chemical process. For the dissolution of LiI, the diagram would show the initial energy of the solid, the energy required to break the ionic bonds, and the final energy of the solution, illustrating the overall energy change and indicating that the process is exothermic due to the net release of energy.
Recommended video:
Guided course
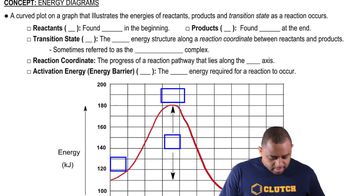
Energy Diagrams
Related Practice
Textbook Question
Textbook Question
When lithium iodide (LiI) is dissolved in water, the solution becomes hotter. b. What can you conclude about the relative magnitudes of the lattice energy of lithium iodide and its heat of hydration?
Textbook Question
When lithium iodide (LiI) is dissolved in water, the solution becomes hotter. d. Why does the solution form? What drives the process?
Textbook Question
Silver nitrate has a lattice energy of -820 kJ/mol and a heat of solution of 22.6 kJ/mol. Calculate the heat of hydration for silver nitrate.
Textbook Question
Use the data to calculate the heats of hydration of lithium chloride and sodium chloride. Which of the two cations, lithium or sodium, has stronger ion–dipole interactions with water? Why?
