In each pair of compounds, pick the one with the higher vapor pressure at a given temperature. Explain your reasoning. b. CH3CH2CH2OH or CH3OH
Ch.11 - Liquids, Solids & Intermolecular Forces

Chapter 11, Problem 44a,c
In each pair of compounds, pick the one with the higher vapor pressure at a given temperature. Explain your reasoning. a. CH4 or CH3Cl c. CH3OH or H2CO
 Verified step by step guidance
Verified step by step guidance1
Identify the intermolecular forces present in each compound. CH_3OH (methanol) has hydrogen bonding due to the presence of an -OH group, while H_2CO (formaldehyde) has dipole-dipole interactions and London dispersion forces.
Recall that stronger intermolecular forces result in lower vapor pressure because more energy is required to overcome these forces and allow molecules to escape into the vapor phase.
Compare the strength of the intermolecular forces: hydrogen bonding in CH_3OH is stronger than the dipole-dipole interactions in H_2CO.
Conclude that the compound with weaker intermolecular forces will have a higher vapor pressure at a given temperature.
Based on the comparison, determine that H_2CO, with weaker dipole-dipole interactions, will have a higher vapor pressure than CH_3OH, which has stronger hydrogen bonding.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Vapor Pressure
Vapor pressure is the pressure exerted by a vapor in equilibrium with its liquid or solid phase at a given temperature. It reflects the tendency of molecules to escape from the liquid or solid state into the gas phase. Compounds with higher vapor pressures at a specific temperature typically have weaker intermolecular forces, allowing more molecules to enter the vapor phase.
Recommended video:
Guided course
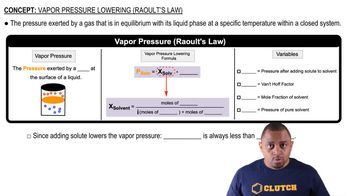
Raoult's Law and Vapor Pressure
Intermolecular Forces
Intermolecular forces are the forces of attraction or repulsion between neighboring particles (atoms, molecules, or ions). These forces include hydrogen bonding, dipole-dipole interactions, and London dispersion forces. Stronger intermolecular forces result in lower vapor pressures, as more energy is required for molecules to escape into the vapor phase.
Recommended video:
Guided course
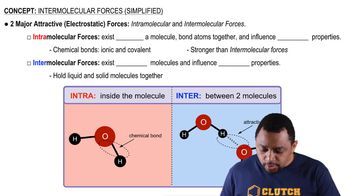
Intermolecular vs Intramolecular Forces
Molecular Structure and Polarity
The molecular structure and polarity of a compound significantly influence its physical properties, including vapor pressure. Polar molecules, like CH3OH (methanol), can form hydrogen bonds, which are strong intermolecular forces. In contrast, H2CO (formaldehyde) is less polar and has weaker dipole interactions, leading to a higher vapor pressure compared to methanol at the same temperature.
Recommended video:
Guided course
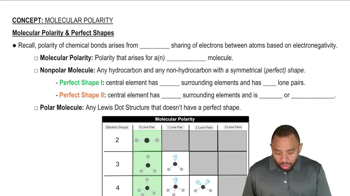
Molecular Polarity
Related Practice
Textbook Question
Textbook Question
Determine whether each pair of compounds forms a homogeneous solution when combined. For those that form homogeneous solutions, indicate the type of forces that are involved. a. CCl4 and H2O b. KCl and H2O c. Br2 and CCl4
Textbook Question
In each pair of compounds, pick the one with the higher boiling point. Explain your reasoning. a. NH3 or CH4 c. CO2 or NO2
Textbook Question
In each pair of compounds, pick the one with the higher boiling point. Explain your reasoning. b. CS2 or CO2
Textbook Question
Determine whether each pair of compounds forms a homogeneous solution when combined. For those that form homogeneous solutions, indicate the type of forces that are involved. d. CH3CH2OH and H2O
