Textbook Question
In each pair of compounds, pick the one with the higher boiling point. Explain your reasoning. c. CH4 or CH3CH3

 Verified step by step guidance
Verified step by step guidance
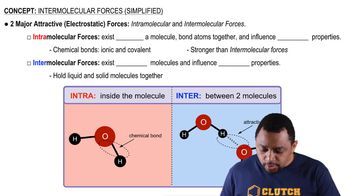
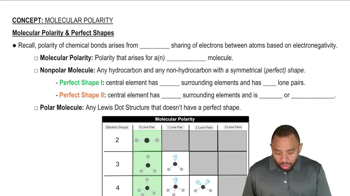

In each pair of compounds, pick the one with the higher boiling point. Explain your reasoning. c. CH4 or CH3CH3
In each pair of compounds, pick the one with the higher vapor pressure at a given temperature. Explain your reasoning. b. CH3CH2CH2OH or CH3OH
In each pair of compounds, pick the one with the higher boiling point. Explain your reasoning. a. CH3OH or CH3SH b. CH3OCH3 or CH3CH2OH
In each pair of compounds, pick the one with the higher boiling point. Explain your reasoning. a. NH3 or CH4 c. CO2 or NO2
In each pair of compounds, pick the one with the higher vapor pressure at a given temperature. Explain your reasoning. a. CH4 or CH3Cl c. CH3OH or H2CO