Which hybridization scheme allows the formation of at least one p bond? sp3, sp2, sp3d2

 Tro 4th Edition
Tro 4th Edition Ch.10 - Chemical Bonding II: Molecular Shapes & Valence Bond Theory
Ch.10 - Chemical Bonding II: Molecular Shapes & Valence Bond Theory Problem 61b
Problem 61bWrite a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7. b. NH3
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
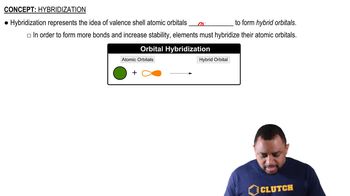
Hybridization
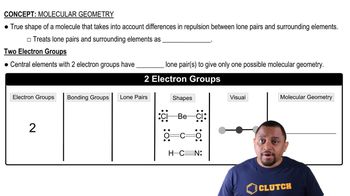
Bonding and Molecular Geometry
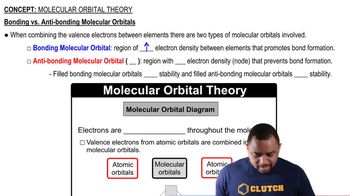
Orbital Overlap Theory
Which hybridization scheme allows the central atom to form more than four bonds? sp3, sp3d, sp2
Write a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7. a. CCl4
Write a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7. c. OF2 d. CO2
Write a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7.a. CH2Br2 b. SO2 c. NF3 d. BF3
Write a hybridization and bonding scheme for each molecule or ion. Sketch the structure, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7.
a. COCl2 (carbon is the central atom)
b. BrF5
c. XeF2