Use molecular orbital theory to predict if each molecule or ion exists in a relatively stable form. a. H22- b. Ne2 c. He22+ d. F22-
Ch.10 - Chemical Bonding II: Molecular Shapes & Valence Bond Theory

All textbooks Tro 4th Edition
Tro 4th Edition Ch.10 - Chemical Bonding II: Molecular Shapes & Valence Bond Theory
Ch.10 - Chemical Bonding II: Molecular Shapes & Valence Bond Theory Problem 80a
Problem 80a
 Tro 4th Edition
Tro 4th Edition Ch.10 - Chemical Bonding II: Molecular Shapes & Valence Bond Theory
Ch.10 - Chemical Bonding II: Molecular Shapes & Valence Bond Theory Problem 80a
Problem 80aChapter 10, Problem 80a
According to MO theory, which molecule or ion has the highest bond order? O2, O2- , O22-
 Verified step by step guidance
Verified step by step guidance1
Identify the molecular orbitals involved in the bonding of O_2 and its ions. For O_2, the relevant molecular orbitals are: \( \sigma_{2s}, \sigma^*_{2s}, \sigma_{2p_z}, \pi_{2p_x} = \pi_{2p_y}, \pi^*_{2p_x} = \pi^*_{2p_y}, \sigma^*_{2p_z} \).
Determine the electron configuration for each species: O_2, O_2^-, and O_2^{2-}. O_2 has 12 valence electrons, O_2^- has 13, and O_2^{2-} has 14.
Calculate the bond order using the formula: \( \text{Bond Order} = \frac{1}{2} (\text{Number of bonding electrons} - \text{Number of antibonding electrons}) \).
For O_2, fill the molecular orbitals with 12 electrons and calculate the bond order. For O_2^-, add one more electron to the antibonding \( \pi^* \) orbitals and calculate the bond order. For O_2^{2-}, add two more electrons to the antibonding \( \pi^* \) orbitals and calculate the bond order.
Compare the bond orders of O_2, O_2^-, and O_2^{2-} to determine which has the highest bond order.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
11mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Molecular Orbital Theory (MO Theory)
Molecular Orbital Theory describes the behavior of electrons in molecules, where atomic orbitals combine to form molecular orbitals that can be occupied by electrons. This theory allows for the prediction of bond order, stability, and magnetic properties of molecules based on the arrangement of electrons in these orbitals.
Recommended video:
Guided course

Molecular Orbital Theory
Bond Order
Bond order is a measure of the number of chemical bonds between a pair of atoms. It is calculated as the difference between the number of bonding and antibonding electrons divided by two. A higher bond order indicates a stronger bond and greater stability of the molecule.
Recommended video:
Guided course

Average Bond Order
Electron Configuration in Diatomic Molecules
The electron configuration of diatomic molecules, such as O2 and its ions, is crucial for determining their bond order. For O2, the configuration is (σ2s)²(σ*2s)²(σ2p)²(π2p)⁴(π*2p)², which helps in calculating the bond order. The addition or removal of electrons in ions like O2- and O2²- alters this configuration and thus affects the bond order.
Recommended video:
Guided course
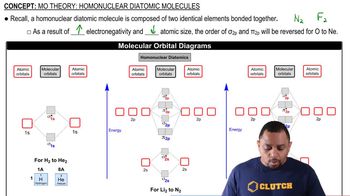
Homonuclear Diatomic Molecules
Related Practice
Textbook Question
Textbook Question
Apply molecular orbital theory to predict if each molecule or ion exists in a relatively stable form. a. C22+ b. Li2 c. Be22+ d. Li22-
Textbook Question
According to MO theory, which molecule or ion has the highest bond order? C2, C2+ , C2- According to MO theory, which molecule or ion has the highest bond energy? According to MO theory, which molecule or ion has the shortest bond length?
Textbook Question
According to MO theory, which molecule or ion has the highest bond energy? O2, O2- , O22-
Textbook Question
According to MO theory, which molecule or ion has the shortest bond length? O2, O2- , O22-
1
views
Textbook Question
Draw an MO energy diagram for CO. (Use the energy ordering of O2.) Predict the bond order and make a sketch of the lowest energy bonding molecular orbital.