Classify each property as physical or chemical. a. the tendency of ethyl alcohol to burn b. the shine on silver c. the odor of paint thinner d. the flammability of propane gas

Classify each change as physical or chemical. a. Sugar burns when heated in a skillet. b. Sugar dissolves in water. c. A platinum ring becomes dull because of continued abrasion. d. A silver surface becomes tarnished after exposure to air for a long period of time.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Physical Change
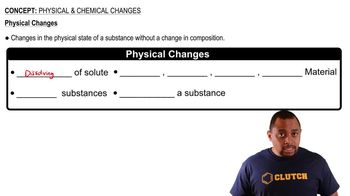
Chemical Change
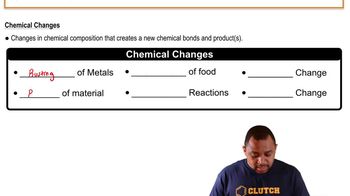
Tarnishing and Abrasion
Classify each property as physical or chemical. a. the boiling point of ethyl alcohol b. the temperature at which dry ice evaporates c. the tendency of iron to rust d. the color of gold
Classify each change as physical or chemical. a. Natural gas burns in a stove. b. The liquid propane in a gas grill evaporates because the valve was left open. c. The liquid propane in a gas grill burns in a flame. d. A bicycle frame rusts on repeated exposure to air and water.
Based on the molecular diagram, classify each change as physical or chemical.
Based on the molecular diagram, classify each change as physical or chemical.
Convert each temperature. a. 32 °F to °C (temperature at which water freezes) b. 77 K to °F (temperature of liquid nitrogen) c. -109 °F to °C (temperature of dry ice) d. 98.6 °F to K (body temperature)
