The warmest temperature ever measured in the United States is 134 °F, recorded on July 10, 1913, in Death Valley, California. Convert that temperature to °C and K.

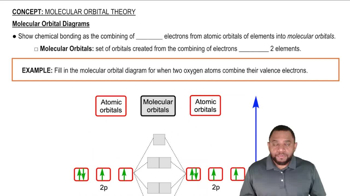
Based on the molecular diagram, classify each change as physical or chemical.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Physical Change
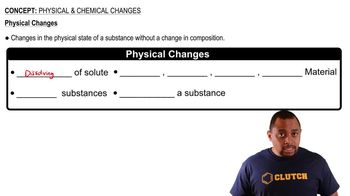
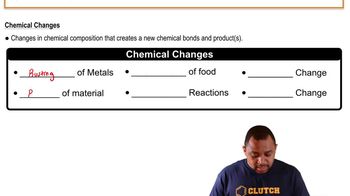
Chemical Change
Molecular Diagram Interpretation
Classify each change as physical or chemical. a. Natural gas burns in a stove. b. The liquid propane in a gas grill evaporates because the valve was left open. c. The liquid propane in a gas grill burns in a flame. d. A bicycle frame rusts on repeated exposure to air and water.
Classify each change as physical or chemical. a. Sugar burns when heated in a skillet. b. Sugar dissolves in water. c. A platinum ring becomes dull because of continued abrasion. d. A silver surface becomes tarnished after exposure to air for a long period of time.
Based on the molecular diagram, classify each change as physical or chemical.
Convert each temperature. a. 32 °F to °C (temperature at which water freezes) b. 77 K to °F (temperature of liquid nitrogen) c. -109 °F to °C (temperature of dry ice) d. 98.6 °F to K (body temperature)
