The following ball-and-stick molecular model is a representation of acetaminophen, the active ingredient in such over-thecounter headache remedies as Tylenol. (Red = O, gray = C, blue = N, ivory = H.) (a) What is the formula of acetaminophen?
Ch.8 - Covalent Compounds: Bonding Theories and Molecular Structure

All textbooks McMurry 8th Edition
McMurry 8th Edition Ch.8 - Covalent Compounds: Bonding Theories and Molecular Structure
Ch.8 - Covalent Compounds: Bonding Theories and Molecular Structure Problem 33a
Problem 33a
 McMurry 8th Edition
McMurry 8th Edition Ch.8 - Covalent Compounds: Bonding Theories and Molecular Structure
Ch.8 - Covalent Compounds: Bonding Theories and Molecular Structure Problem 33a
Problem 33aChapter 8, Problem 33a
The following ball-and-stick molecular model is a representation of thalidomide, a drug that causes birth defects when taken by expectant mothers but is valuable for its use against leprosy. The lines indicate only the connections between atoms, not whether the bonds are single, double, or triple. 1Red = O, gray = C, blue = N, ivory = H.2 (a) What is the formula of thalidomide?
 Verified step by step guidance
Verified step by step guidance1
Identify the colors representing each type of atom in the ball-and-stick model: Red for Oxygen (O), Gray for Carbon (C), Blue for Nitrogen (N), and Ivory for Hydrogen (H).
Count the number of each type of atom in the model. Carefully examine the model to ensure all atoms are accounted for.
Write down the chemical symbols for each type of atom, followed by the number of atoms of that type in the molecule. This will form the molecular formula.
Ensure that the molecular formula is written in the standard order: Carbon (C) first, followed by Hydrogen (H), and then the other elements in alphabetical order.
Double-check the counts and the order of elements to ensure the molecular formula accurately represents the model.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Molecular Formula
A molecular formula represents the number and types of atoms in a molecule. It is expressed using chemical symbols and subscripts, indicating how many of each atom are present. For example, the molecular formula for water is H2O, meaning it contains two hydrogen atoms and one oxygen atom. Understanding how to derive a molecular formula from a molecular model is essential for identifying the composition of a compound.
Recommended video:
Guided course

Determining Molecular Formulas
Chemical Structure
Chemical structure refers to the arrangement of atoms within a molecule, including the types of bonds (single, double, or triple) between them. In a ball-and-stick model, different colors represent different elements, and the sticks represent bonds. Recognizing the structure helps in understanding the properties and reactivity of the molecule, which is crucial for determining its formula and function.
Recommended video:
Guided course

Chemical Properties
Functional Groups
Functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. In organic chemistry, common functional groups include hydroxyl (-OH), carboxyl (-COOH), and amine (-NH2) groups. Identifying functional groups in a compound like thalidomide can provide insights into its biological activity and potential effects, such as its teratogenic properties.
Recommended video:
Guided course
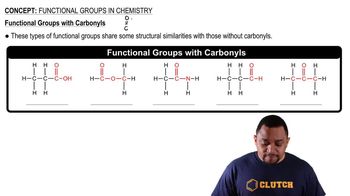
Carbonyl Functional Groups
Related Practice
Textbook Question
1
views
Textbook Question
The following ball-and-stick molecular model is a representationof acetaminophen, the active ingredient in such over-thecounterheadache remedies as Tylenol. 1Red = O, gray = C,blue = N, ivory = H.2(c) What is the geometry around each carbon?
1
views
Textbook Question
The following ball-and-stick molecular model is a representation of acetaminophen, the active ingredient in such over-thecounter headache remedies as Tylenol. (Red = O, gray = C, blue = N, ivory = H.) (b) Indicate the positions of the multiple bonds in acetaminophen.
Textbook Question
Ethyl acetate, CH3CO2CH2CH3, is commonly used as a solvent and nail-polish remover. Look at the following electrostatic potential map of ethyl acetate, and explain the observed polarity.
1
views
Textbook Question
Two dichloroethylene molecules with the same chemical formula 1C2H2Cl22, but different arrangements of atoms are shown. (b) Which form of dichloroethylene has a dipole moment of 2.39 D, and which has a dipole moment of 0.00 D?
1
views
1
rank
Textbook Question
What geometric arrangement of charge clouds do you expect for atoms that have the following number of charge clouds? (a) 3 (b) 5 (c) 2 (d) 6
1
views