Textbook Question
Phosphorus can have several different oxidation numbers ranging in value from -3 to +5. (b) Based on oxidation numbers, which phosphorus oxide compound from part (a) was formed by combustion with a limited supply of oxygen?

 Verified step by step guidance
Verified step by step guidance


Phosphorus can have several different oxidation numbers ranging in value from -3 to +5. (b) Based on oxidation numbers, which phosphorus oxide compound from part (a) was formed by combustion with a limited supply of oxygen?
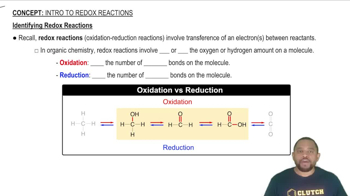
Which element is oxidized and which is reduced in each of the following reactions? (a)
Which element is oxidized and which is reduced in each of the following reactions? (b)