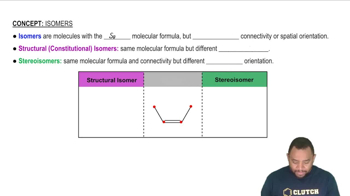
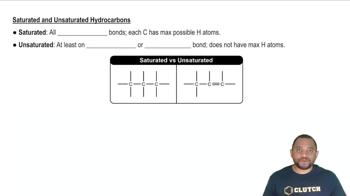
Textbook Question
Butane, the fuel used in disposable lighters, has the formula C4H10. The carbon atoms are connected in the sequence C-C-C-C, and each carbon has four covalent bonds. Draw the structural formula of butane.

 Verified step by step guidance
Verified step by step guidance